Has agricultural biotechnology finally turned a corner?

Policy proposals
- Governments, NGOs and other civil society representatives should engage in a comprehensive public debate on how advances in agbiotech, can benefit smallholders in developing countries by producing more sustainable food systems and improving nutrition in view of changing climate and resource scarcity. In particular genome editing in crop and livestock improvement should be accommodated.
- Governments across the world should urgently address the issue of how biotech-derived crops are regulated, especially in view of recent developments in genome editing for crop and livestock improvement
- A public sector led initiative, possibly coordinated by FAO, should be set up to investigate the feasibility of developing open-source biotechnologies (especially genome editing) for use in public-good applications in developing countries.
- Increasing support should be given to Public Private Partnerships (PPPs) to increase agbiotech innovation. This would include support for the public R&D sector, particularly in developing countries, where there is enormous scope for adaptation of bioscience advances. It would also mean more support, not the least with incubation services, for PPPs to target low profit markets with high social impact, so contributing to sustainable development.
Introduction
Agricultural biotechnology, or agbiotech, encompasses a diverse range of biologically based methods that have been applied to agricultural improvement since the beginning of modern scientific breeding in the early 20th century (1).
As we move through the second decade of the 21st century, we face considerable challenges such as population growth, food insecurity, climate change, fresh water scarcity, and the emergence of new pests and diseases (1,2). It is therefore more important than ever that we are able to use these biological tools to increase the yield and quality of our crop and livestock resources. It is also vital that this goal is achieved in a manner that is both environmentally appropriate and sustainable in the long term, especially in the context of the requirement for continued intensive food production by future generations (2).
Agbiotech is often equated with so-called GM (genetic manipulation) technologies. However, many bio-based technologies were being widely used in agriculture long before the first GM bacteria were created in the 1970s, or the first GM plants in the 1980s
Examples of such lab-based, but non-GM, biotechnologies include the use of tissue culture for micropropagation, the creation of hybrid plants between and within species (e.g. via embryo rescue), the manufacture of doubled haploids,
and the use of induced mutagenesis involving chemicals or radiation to create novel crop phenotypes.
Other examples include use of fermentation to modify foods, DNA markers to select suitable variants in large populations, and reproductive technologies such as cloning. None of these biotechnologies are legally defined as ‘GM’ and most of them have been used without controversy for many decades (3).
Interestingly, GM methods are now used routinely and without controversy in a host of medical applications, such as the production of life-saving recombinant therapeutic products including human insulin and blood clotting factors.
In contrast, however, the use of GM technologies in agriculture has caused substantial (if arguably unwarranted) public concern.
This has, to some extent, tarnished the entire field of agricultural biotechnology, especially in some parts of Europe where field trials of new crop cultivars are sometimes still subject to vandalism by anti-GM campaigners. In this context, it was telling that at a high level conference on agbiotech convened in Mexico in 2010 by the United Nations Food and Agriculture Organization, only one European country (The Netherlands) bothered to send a delegation.
In contrast, dozens of developing country delegations attended as well as other major food-exporting nations such as Canada and the USA (4). At the time a group of European agricultural scientists expressed their concerns as follows:
“We wish to express our concern and dismay at the apparent lack of intergovernmental engagement by European governments regarding the proven positive roles of modern biotechnologies as key tools supporting efforts to address the issue of food security, especially in developing countries. This was shown clearly by the failure of 26 of the 27 members states of the European Union to send any official government delegations to participate and engage in the recent United Nations Food and Agriculture Organization (FAO; Rome) intergovernmental conference (ABDC-10) on ‘Agricultural biotechnologies in developing countries’ (http://www.fao.org/biotech/abdc/en). The Netherlands was the only EU member state to send an official government delegate to ABDC-10.” (4)
Indeed, over the past twenty years since the first large-scale releases of GM crops took place in the USA in 1996, there has been a distinct scepticism about agbiotech, and especially GM crops, in Europe. This scepticism was also manifested in some developing countries where GM crops have been regarded in some quarters as foreign threats to indigenous crops or even as evidence of a neo-colonialist drive by a few multinational corporations to take over the food chain (5,6). In a few cases, much needed food aid was rejected in Africa, simply because it included GM crop products from the USA that were regarded with unwarranted suspicion by local politicians (7).
In this article, we argue that there are now signs of significant changes in global attitudes to agbiotech that have emerged as a result of increasing engagement by developing countries. This has been coupled with some dramatic advances that have the potential to democratise the use of these technologies.
Indeed it can be argued that agbiotech has recently made decisive strides in terms of its broad utility, practicality, and public acceptability across much of the world. Unfortunately, however, awareness of these developments in Europe continues to lag behind much of the rest of the world and the region is in danger of becoming a backwater in the application of some forms of modern agriculture.
These fears are also addressed by a report by the European Academies Science Advisory Council (EASAC) (8). EASAC makes the point that owing to stifling regulations countries in Europe, limited R&D funding and innovation investments in agbiotech.
This made these countries unable to fully use the advances of modern biosciences to develop more productive, resource efficient and climate smart agricultural systems.
The FAO Symposium on agbiotech, February 2016
From 15-17 February, 2016, some 400 scientists, government officials, private sector and civil society representatives gathered at the headquarters of the United Nations Food and Agriculture Organization in Rome to discuss “The Role of Agricultural Biotechnologies in Sustainable Food Systems and Nutrition” (9).
This landmark symposium was a follow up from the FAO agbiotech meeting in Mexico in 2010 (see Introduction above).
The key question at the 2016 meeting was to discuss to what extent agricultural biotechnologies could assist and benefit smallholders in developing sustainable food systems and improving nutrition in the context of climate change.
The meeting was also a forum for discussion of new developments in the creation of new biotechnologies and the practical implementation of existing biotechnologies in countries ranging from Bangladesh to Brazil.
As stated in the plenary session by José Graziano da Silva, Director-General, FAO, “Given the magnitude of the challenge, we must use a broad portfolio of tools to fight malnutrition and achieving sustainable agriculture”.
It was repeatedly emphasized by participants that agbiotech should
be regarded as a large and rapidly expanding toolbox of bioscience technologies, ranging from controversial and fast evolving techniques, such as genome editing and conventional genetic modification (GM) to less controversial techniques such as mutagenesis and tissue culture (non-GM technologies).
As stated on the FAO website: “The international symposium will explore how the application of science and technology, particularly agricultural biotechnologies, can benefit smallholders in developing sustainable food systems and improving nutrition in the context of climate change.
The symposium takes a multisectoral approach, covering the crop, livestock, forestry and fishery sectors. It also aims to cover the wide spectrum of available biotechnologies, including microbial food fermentation, tissue culture in plants, reproductive technologies in livestock, use of molecular markers, genetic modification and other technologies.”
Although there were some delegates who disputed the relevance of agbiotech for small farmers and who continued to stress the corporatist origins of GM technologies, it was striking that most developing country delegates were firmly in favour of the use of agbiotech for the improvement of both crop and livestock husbandry. This was especially true for delegates from Africa where the past few years have witnessed the release of numerous biotech-improved crop cultivars that were developed using both GM and non-GM technologies.
In the next section of this article, three case studies on uptake of agbiotech in developing countries that featured at the symposium will be presented. For interested readers, the complete Powerpoint presentations from the meeting are available at: http://www.fao.org/3/a-bc787e.pdf and most of the verbal presentations are also available as webcasts at: http://www.fao.org/about/meetings/agribiotechs-symposium/webcasting/en/.
Case studies on uptake of agbiotech in developing countries
1. Brinjal in Bangladesh: breaking the impasse on GM crop acceptance?
Over recent decades, Bangladesh made enormous strides in the use of modern agricultural practices to the extent that this once famine-prone and overcrowded nation is now self-sufficient in key staple crops such as rice (10, 11).
In her presentation to the FAO Symposium, Begum Matia Chowdhury, the long-serving Minister of Agriculture in Bangladesh, outlined some of the impressive recent achievements in her country and especially the introduction of a cultivar of GM brinjal (also called zucchini or eggplant) that is engineered to be tolerant to attack by certain insect pests. This was achieved through the expression of the naturally occurring bacterial insecticide Bt in the brinjal plant.
The Bt insecticide originates from Bacillus thuringiensis, which is a soil bacterium that produces so-called Bt proteins that are toxic to certain groups of insects.
To date, researchers have taken the gene(s) encoding one or more of the >30 Bt toxins and introduced them into crops such as soybean and cotton to produce cultivars that can protect themselves from larval attack.
While there have been several examples of widespread smallholder adoption of GM cash crops, most notably Bt cotton in India and Africa, several promising subsistence GM crop candidates have faced lengthy delays. However, as noted by Begum Chowdhury, Bangladesh approved Bt brinjal/eggplant for planting in 2013 (12). In 2014 commercialization was initiated via a Public-Private Partnership (PPP) when a total of 120 farmers planted 12 hectares. This followed strong political support from the government, with leadership from Ministry of Agriculture, and close collaboration with farmer groups and private sector breeders.
Despite the expected adverse publicity from anti-GM campaigners, the introduction of Bt brinjal in Bangladesh appears to have been a qualified success, decreasing the use of chemical insecticides, and improving crop productivity and farm profitability (13).
This approval of GM Bt brinjal by Bangladesh is important in that it has broken the impasse experienced in trying to gain approval for commercialization of the introduction of the same crop in India and the Philippines.
It also serves as a possible model for other small poor countries.
Two other developing countries in Asia, Vietnam and Indonesia, also approved cultivation of GM crops in 2014 for commercialization in 2015 (14).
Vietnam approved Bt maize and Indonesia approved a drought tolerant sugarcane for food, whilst approval for feed is pending; 50 hectares of sugarcane were planted in 2014 for planned commercialization in 2015.
In 2014, it is estimated that approximately 18 million farmers grew GM crops, about 90%, or 16.5 million, were small farmers in developing countries.
In addition to economic gains, farmers benefited enormously from at least a 50% reduction in the number of insecticide applications, thereby reducing farmer exposure to insecticides, and importantly it contributed to a more sustainable environment and better quality of life.
It is noteworthy that many of these
GM crops were introduced via Public-Private Partnerships (PPPs) rather than being simply for-profit ventures by global multinational corporations.
The importance of PPPs in the roll out of new crop cultivars has been discussed in recent reports from FAO (15) and the Joint Research Centre of the European commission (16).
2. EMBRAPA in Brazil: PPP-led GM crop development
EMBRAPA (Empresa Brasileira de Pesquisa Agropecuária or the Brazilian Agricultural Research Corporation) is the major public sector agricultural R&D organization in Brazil.
With an annual budget of US$1 billion, EMBRAPA has been especially active in fostering PPPs in agbiotech.
EMBRAPA is also one of the few public bodies in the world to have a long term programme for development and commercialisation of GM crops.
It initially focused mainly on soybeans, which are now grown by millions of Brazilian farmers ranging from smallholders to large international combines. In 2014, Brazil commercially planted GM soybeans with insect resistance and herbicide tolerance on 5.2 million hectares (Mha).
This was a substantial increase from 2.2 Mha in 2013 (14). The expansion of soybean production in Brazil has been a driver of economic growth and increased farm incomes, although it has also been widely discussed in terms of its putative environmental impact (17, 18).
More recently EMBRAPA has further widened the scope of its GM crop portfolio. In 2015, it gained approval to commercialize a locally developed cultivar of pinto beans with resistance to the devastating golden mosaic virus, planned for release in 2016.
EMBRAPA has also developed a novel herbicide tolerant soybean via a PPP with BASF. This new soybean cultivar is currently awaiting EU import approval prior to planned commercialization later in 2016 or 2017. EMBRAPA is also developing GM folate-fortified lettuce and drought resistant sugarcane (14).
EMBRAPA is an example of a large state enterprise that has taken the lead in innovative biotech crop development, with PPP engagement where appropriate.
While it was initially focused mainly on the major commercial crop, soybean, EMBRAPA has now started to produce GM crops aimed more at smallholders and internal markets rather than commodity crops for export.
3. Emergence of agbiotech Public-Private Partnerships (PPPs) in Africa
Over the past decade there have been numerous PPP ventures in Africa that have focused on both GM and non-GM crops aimed at smallholders (19, 20).
For example, Cameroon, Egypt, Ghana, Kenya, Malawi, Nigeria, and Uganda have deployed agbiotech breeding tools and conducted field trials on the following broad range of staple crops (2): rice, maize, wheat, sorghum, bananas, cassava, and sweet potato (orphan crops include many developing country staples that have been relatively neglected in terms of modern breeding activities).
African GM crops are often the result of major PPP efforts that include development of cultivars with increased drought tolerance, better storage properties, improved disease resistance and nutritional characteristics, such as biofortfied cassava.
These crops are of great interest to small-scale farmers and also for the food insecure. Despite their obvious benefits, it will take many years for these GM crops to reach small-scale farmers because of the regulatory hurdles, inefficient technology dissemination pathways and weak seed markets.
The Water Efficient Maize for Africa (WEMA) is, however, expected to deliver its first GM drought tolerant maize with Bt insect resistance in South Africa as early as in 2017, followed by Kenya and Uganda, and then by Mozambique and Tanzania, subject to regulatory approval (21).
Bt cotton is grown at a commercial scale in South Africa, Burkina Faso and Sudan. In 2015 there was an apparent setback to GM crop cultivation in one African country when Burkina Faso announced that it would be starting a phased reduction of Bt cotton cultivation (22-24).
The cultivar had been developed by Monsanto and its introduction was strongly backed by the government of Burkina Faso. The crop had been popular among farmers as it was less labour intensive and required fewer inputs than conventional cotton.
Cotton yields were good but it turned out that the Bt cotton cultivar used produced fibre that was traded at a discount to that of other West African origins so the cotton processors had reduced incomes (24).
The problems with Bt cotton in Burkina Faso are therefore not related to the Bt trait or GM technology per se. Rather they are the consequence of what were arguably some poor choices of plant material during the breeding process. Such mistakes can be relatively easily rectified but this will take several years and a continued commitment by all parties to the project, which may not be forthcoming in the present case.
The experience in Burkina Faso, points to the importance for African countries to build their own capacity and PPPs enabling them to develop biotechnology crops adopted to the needs of their local farmers and local markets.
The Burkina Faso case
notwithstanding, over the past two years there has been a distinct improvement in state engagement with biotech related PPPs in much of Africa.
The major lessons from recent agbiotech PPP experiences are that success is dependent on a full commitment by host countries, appropriate regulatory systems, and the sustained participation of all partners, especially smallholders, over the entire duration of what can be complex and long term ventures.
Prospects for sustained agricultural improvement in Africa, including but by no means limited to biotech-related crops, are now particularly bright, as outlined in recent articles in the Economist (25) and elsewhere (26, 27).
The potential impact of new genome editing biotechnologies
During the last few years, and especially in 2015, there has been a veritable revolution in genetic technologies with the development of genome editing methods such as CRISPR and TALENs (28-31).
Genome editing technologies are already being used for the improvement of both livestock (33) and crops (34-37).
In terms of crop breeding, this means that it will soon be possible to progress from the random insertion of single or a small numbers of genes into a genome (as in traditional GM) to the highly precise insertion into a defined location of large numbers of genes, chromosome segments or pseudo-segments encoding entire metabolic pathways into virtually any plant species (3).
Another significant aspect of the new genome editing systems is that, in some cases, it may be virtually impossible to detect any resultant modifications in a genome.
This is in contrast to conventional late-20th century GM methods where the presence of novel DNA can be readily detected (28, 33). Therefore some new genome edited plants may not carry any proof whether they were produced either via a GM-type technology or via one of several non-GM technologies that could have been used instead.
This development has the potential to undermine the entire framework that is currently used for the regulation of GMOs because, for example, it would become impossible to distinguish between a new plant cultivar that has arisen via spontaneous mutation and a non-GM plant produced via deliberate mutagenesis in the lab or a GM plant that has been deliberately modified by genome editing.
It has been argued that these new technologies could make some of the current GM methods (and their regulation) obsolete and, as discussed below, there are already calls that, in some cases, organisms altered by genome editing should not be characterized as GMOs (33, 34).
Genome editing can considerably widen the range of traits (especially smallholder relevant traits in hitherto orphan crops) to be altered much more rapidly and cheaply than was hitherto possible. The user friendliness and the comparatively low cost of the genome editing technologies may also enable low-income countries to leapfrog into the world of precision breeding.
This provides a golden opportunity for the emergence of a new generation of PPPs aimed specifically at improving low profit smallholder agriculture as we face up to increasing food security challenges across the world.
A sluggish response to agbiotech innovations in Europe
Legislators in Europe have been slow to respond to the developments in genome editing technologies that have emerged over the past decade, but especially since 2014.
As early as 2007 the European Commission appointed an expert panel to advise it on the ever-expanding plant-breeding toolbox but the panel’s report, submitted in 2012, was never published.
There have been many scientific publications that have highlighted the need for a reconsideration of the EU regulatory environment for GM crops. Nevertheless, even now, in 2016, there is little evidence of awareness amongst policymakers of how these new technologies and other advances in the rapidly expanding field of biosciences are changing the conditions for breeding and crop production.
There is an urgent need to revise, adjust and modernize biosafety regulations that were mostly designed in the early 1990s (36-39) in order to take account new technologies and new knowledge such as, but by no means limited to, genome editing (29, 40-44).
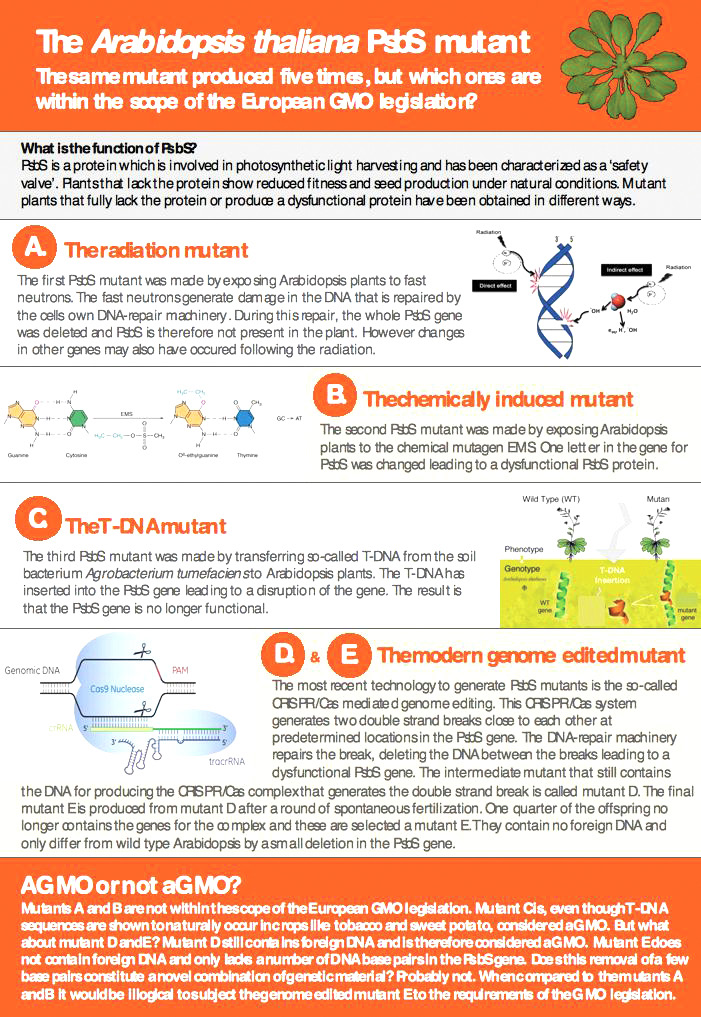
In late 2015, an assessment about whether plants created via the CRISPR/Cas9 system should be classified as ‘GM’ was published by the Swedish Board of Agriculture (SBA) (45). The conclusion was that while individual cases might differ, the Arabidopsis plants examined in their study should not be regarded as GMOs (see also Figure 5).
It was stated that: “The SBA makes the interpretation that those plants in the description mutated by CRISPR / Cas9 and which do not contain any foreign DNA are exempted from the GM legislation.” (45)
Despite such clear and explicit evidence-based verdicts from this and
other expert studies, it is difficult to be optimistic that European regulations will be modified in the near future to recognise the new scientific landscape arising from genome editing and related technologies.
In contrast, in the USA there are intensive consultations between scientists, policymakers, and legislators on how to amend the current framework regulating the use advanced agbiotech so that it ensures safety and sustainability but at the same time continues to promote innovation and deployment of the fast advances in biosciences.
Given the enormous potential of genome editing for developing country crops (46), it seems more likely that many developing countries will tend to model their own regulatory systems on US systems, enabling technologies to be disseminated to address agronomic challenges and development opportunities.
Another way in which the context of the GM debate has changed GM-related methods since the 1970s is the growing evidence that gene transfer between unrelated species in commonplace in the ‘natural’ world.
Prior to the 2000s, it was believed that the transfer of functional DNA sequences between different species was limited to very simple organisms such as bacteria. In contrast, it was (and still is in many quarters) believed that the genomes of more complex organisms such as higher plants and animals were relatively fixed within individual species and that the insertion of exogenous DNA was therefore ‘unnatural’. This perspective has now entirely changed and there are many recently discovered examples of gene transfer between completely unrelated plants, animals and microbes leading to the creation of new genotypes and phenotypes that sometime have evolutionarily significant selective advantages (47, 48).
In addition to naturally occurring DNA transfer between species, there are reports of soil bacteria acting as ‘natural’ genetic engineers by inserting novel genes into plants, including crops (49). The progeny of such plants will inherit the inserted bacterial genes and pass them on in turn to their descendants.
This results in the non-human mediated creation of a completely new transgenic plant cultivar – in effect a ‘naturally occurring’ GMO. In the case reported by Kyndt et al. (49), a transgenic version of the sweet potato plant with four extra bacterial genes was found. Sweet potatoes have been grown by farmers as a food crop for millennia but, interestingly, wild relatives of sweet potato did not contain the bacterial genes. This means that humans have inadvertently selected a transgenic version of the sweet potato to grow as a crop in preference to non-transgenic versions. In the light of these discoveries, it is difficult to maintain the stance that GMOs themselves, and even the creation of GMOs (whether by humans or bacteria), are completely aberrant and fundamentally ‘unnatural’ instances of human interference in the wider biological world.
In view of the growing scientific evidence about gene transfer in the ‘natural’ world and the emergence of radical, and sometimes undetectable, methods of genome editing it is arguably timely for a fundamental reappraisal of the nature of GM technologies to be undertaken.
Such discussions are already underway in the Americas and elsewhere, but Europe continues to lag behind with a poor engagement and chronic lack of leadership in the international discourse on agbiotech, and especially its implementation and regulation (4).
Unless this situation changes radically in the next few years, we may face a situation in which the rest of the world, including Africa, benefits from new breeding technologies and new genomic advances (some initiated by European researchers) while Europe itself becomes a stagnant backwater in
terms of applied crop breeding and biotechnology.
In essence it would mean that Europe might miss the opportunity to use this rapid and increasingly powerful toolbox of modern biosciences to develop productive, resource-efficient sustainable crop production systems for food, feed and agro-industrial products.
Conclusions and future prospects
Over recent decades there has been a somewhat polarised and largely sterile debate about several aspects of agbiotech, particularly relating to intensive farming practices and the use of GM technologies for crop cultivation.
There has also been a great deal of pessimism about developing country agriculture, especially in Sub Saharan Africa. For a variety of reasons the region was unable to match the truly spectacular yield gains made in the rest of the developing world as part of the momentous Green Revolution in the 20th century.
More recently, however, the have been signs that the rhetoric may be changing. ‘Conventional’ GM crops (i.e. those created using the original 20th century technologies of transgenesis via recombinant DNA methods) are now being increasingly deployed around the world, often as part of public sector-led PPPs aimed at smallholders.
At present, the GM trait and seed market is still heavily concentrated among a few multinational actors and just four major global feed and commodity crops, maize, soybeans, rapeseed and cotton.
However, we are now witnessing, not least in Africa and Asia, the development of a greater diversity of GM and non-GM crops, developed by a wider set of actors. These efforts will hopefully increase both the yield and quality of a wide variety of crops that would benefit a broad set of farmers and consumers and also the environment in the years to come (25-27). This may be a new dawn for both industrialised and developing countries, making a wider use of the burgeoning toolkit now available for breeders and agronomists in addressing a set of environmental and development challenges.
In particular, the use of genome editing and genomics technologies has the scope to greatly reduce the cost, and effectiveness and to lower the entrance barriers for the use of advanced biotechnologies.
These should increase crop yield, quality and crop diversity. Moreover, they should lead to more resource efficient crop production systems tolerant to both increasing disease pressure and climatic stress. These new genetic technologies may eventually make much of the current ‘conventional’ GM-based crop improvement technologies and its risk assessment & regulation obsolete.
There are already calls that organisms altered by genome editing should not be characterised as GMOs.
Genome editing can considerably widen the range of traits (especially smallholder-relevant traits in hitherto orphan crops) that can be addressed through gene modification and these will be altered much more rapidly and cheaply than was hitherto possible.
There is an urgent need to accelerate capacity building in all forms of agbiotech and related public outreach in all countries. This provides a golden opportunity for the emergence of a new generation of innovative PPPs and new agbiotech paradigms aimed specifically at improving smallholder agriculture as we face up to increasing food security challenges across the world.
There is also, however, a common agreement that agbiotech is not a means in itself, but has to be combined with other disciplines and drivers such as agroecology, functional markets and value chains, to be fully effective.
References
1. Murphy DJ (2011) Current Status and Options for Crop Biotechnologies in Developing Countries, in Biotechnologies for Agricultural Development, pp 6-24, FAO, Rome, available online: http://www.fao.org/docrep/014/i2300e/i2300e.pdf http://www.fao.org/docrep/014/i2300e/i2300e00.htm
2. FAO, IFAD & WFP (2015) The State of Food Insecurity in the World 2015. Meeting the 2015 international hunger targets: taking stock of uneven progress. FAO, Rome.
3. Murphy DJ (2016) The potential of biosciences for agricultural improvement: looking forward to 2050, In Virgin I & Morris J, Eds, Creating Sustainable Bioeconomies, The Bioscience Revolution in Europe and Africa, Routledge, https://www.routledge.com/products/9781138818538
4. Atanassov A et al (2010) 1 out of 27—European politicians score poorly in agbiotech, Nature Biotechnology 28, 551-552
5. Gridneff I (2012) GM for Uganda. Feeding the world or the greedy? The Global Mail, available online: http://tgm-archive.github.io/gmo-food/ugandas-choice.html
6. Specter M (2014) Seeds of Doubt, The New Yorker, available online: http://www.newyorker.com/magazine/2014/08/25/seeds-of-doubt
7. Annear CM (2004) “GM or Death”: Food and Choice in Zambia, Gastronomica 4:2, available online: http://www.gastronomica.org/gm-death-food-choice-zambia/
8. European Agencies Science Advisory Council (EASAC) (2013) Planting the future: opportunities and challenges for using crop genetic improvement technologies for sustainable agriculture EASAC policy report 21, available online: http://www.easac.eu/fileadmin/Reports/Planting_the_Future/EASAC_Planting_the_Future_FULL_REPORT.pdf
9. UNFAO (2016) FAO Symposium on ‘The Role of Agricultural Biotechnologies in Sustainable Food Systems and Nutrition’, FAO, Rome, Italy, 15-17 February 2016, available online: http://www.fao.org/about/meetings/agribiotechs-symposium/en/
10. McKee D (2012) Bangladesh – road to self-sufficiency, available online: http://www.davidmckee.org/2012/11/17/bangladesh-road-to-self-sufficiency/
11. FAO (2011) Bangladesh and FAO. Success stories and achievements, FAO, Rome, Italy, available online: http://www.fao.org/fileadmin/user_upload/faobd/docs/Achievements/Bangladesh_edoc_final_en.pdf
12. Mohindru SC (2013) Bangladesh Approves Cultivation of Genetically Modified Eggplant, Wall Street Journal, Nov 22, 2013, available online: http://www.wsj.com/articles/SB10001424052702303653004579213572545045240
13. Entine J (2015) As success grows for Bangladesh’s Bt brinjal (eggplant), Mae-Wan Ho renews GMO disinformation campaign, Genetic Literacy Project, April 27, 2015, available online: https://www.geneticliteracyproject.org/2015/04/27/as-success-grows-for-bangladeshs-bt-brinjal-eggplant-mae-won-ho-renews-gmo-disinformation-campaign/
14. James C (2014) Global Status of Commercialized Biotech/GM Crops: 2014. ISAAA Brief No. 49. ISAAA: Ithaca, NY, available online: http://www.isaaa.org/resources/publications/briefs/49/
15. FAO (2015) Fourth private sector partnerships dialogue: Inclusive finance and investment models in agriculture, FAO, Rome, Italy, available online: www.fao.org/3/a-az567e.pdf
16. Murphy DJ (2014) Modern plant breeding technologies and PPPs, In: Proceedings of Workshop on public-private partnerships in plant breeding, Lusser M, Ed, Joint Research Centre, Publications Office, European Union, Luxembourg, available online: http://publications.jrc.ec.europa.eu/repository/bitstream/111111111/31968/1/ipts%20jrc%2088788%20%28online%29%20final.pdf
17. WWF (2014) The Growth of Soy: Impacts and Solutions. WWF International, Gland, Switzerland, available online: http://wwf.panda.org/what_we_do/footprint/agriculture/soy/soyreport/
18. Barona E (2010) The role of pasture and soybean in deforestation of the Brazilian Amazon, Environ. Res. Lett. 5 024002, available online: http://iopscience.iop.org/article/10.1088/1748-9326/5/2/024002/pdf
19. Kameri-Mbote P et al (2001) Public / Private Partnerships for Biotechnology in Africa: The Future Agenda, Africa Centre for Technology Studies, Nairobi, available online: http://www.ielrc.org/content/a0107.pdf
20. Bailey R et al (2014) On trial: agricultural biotechnology in Africa, Chatham House, available online: https://www.chathamhouse.org/sites/files/chathamhouse/field/field_document/20140716BiotechAfrica.pdf?dm_i=1TY5,2N30J,BHZLN4,9NERT,1
21. WEMA website (2016) http://wema.aatf-africa.org/about-wema-project
22. Schnurr M (2016) Bt Cotton in Burkina Faso, 2015 Conference: Can Genetically Modified Crops Help the Poor? available online: http://gmosandpoverty.com/bt-cotton-in-burkina-faso/
23. Dowd-Uribe B and Schnurr M (2016) Lessons to be learnt from Burkina Faso’s decision to drop GM cotton, The Conversation, Feb 2 2016, available online: http://theconversation.com/lessons-to-be-learnt-from-burkina-fasos-decision-to-drop-gm-cotton-53906
24. Burkina Faso plans to decrease planting area of Bt cotton, AgroNews, June 23, 2015, available online: http://news.agropages.com/News/NewsDetail---15132.htm
25. Economist (2016) African agriculture, a green revolution, March 12, 23-25
26. Sanchez PA (2015) En route to plentiful food production in Africa, Nature Plants 1, 1-2
27. Cernansky, R. 2015. Super vegetables. Nature 522, pp.146-148
28. Ledford H (2015) CRISPR, the disruptor, Nature 522, 20-24
29. Ainsworth C. (2015). Agriculture: A new breed of edits, Nature 528, S14-15
30. Davis E. (2015). Genome Editing: Which Should I Choose, TALEN or CRISPR? Technical Note, GeneCopoeia Inc, available online: www.genecopoeia.com
31. Hsu PD, et al. (2014) Development and applications of CRISPR-Cas9 for genome engineering, Cell 157, 1262-1278
32. Petherick A et al (2015) Genome editing, Nature 528 S1-S48
33. Lunshof J (2015) Regulate gene editing in wild animals, Nature 521, 127
34. Mao Y, et al. (2013) Application of the CRISPR-Cas system for efficient genome engineering in plants, Molecular Plant 6, 2008-2011
35. Ricroch AE and Hénard-Damave MC (2015) Next biotech plants: new traits, crops, developers and technologies for addressing global challenges, Critical Reviews in Biotechnology doi:10.3109/07388551.2015.1004521
36. Zhang H et al (2014) The CRISPR/Cas9 system produces specific and homozygous targeted gene editing in rice in one generation, Plant Biotechnology Journal 12:797-807
37. Abbott, A. (2015). Europe’s genetically edited plants stuck in legal limbo. Scientists frustrated at delay in deciding if GM regulations apply to precision gene editing. Nature 528, 319-320
38. Anon (2015a) Seeds of change. The European Union faces a fresh battle over next-generation plant-breeding techniques, editorial, Nature 520, 131-132
39. Anon (2015b). Crop conundrum. The EU should decide definitively whether gene-edited plants are covered by GM laws. Nature 528, 307-308
40, ACRE (2012) ACRE advice: New techniques used in plant breeding, Advisory Committee on Releases to the Environment, UK, available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/239542/new-techniques-used-in-plant-breeding.pdf
41. Ainsworth, C. (2015). Agriculture: A new breed of edits. Nature 528, S14-15
42. Wolt JD et al (2016) The Regulatory Status of Genome-edited Crops, Plant Biotechnology Journal 14, 510-518
43. Huang S et al (2016) A proposed regulatory framework for genome-edited crops, Nature Genetics 48, 109-111
44. Roberts AF, et al. (2015) Biosafety research for non-target organism risk assessment of RNAi-based GE plants, Frontiers in Plant Science 6:958
45. Eklöf, S. (2015). CRISPR/Cas9 mutated Arabidopsis, Swedish Board of Agriculture, Jönköping, available online: http://www.umu.se/digitalAssets/171/171717_backgroud-psbs.pdf http://www.upsc.se/documents/Information_on_interpretation_on_CRISPR_Cas9_mutated_plants_Final.pdf
46. Erbentraut, J. and Shapiro, L. (2015) The Genetic Revolution Could Curb World Hunger And Pesticide Use, HiffPost Science, 12 Dec 2015
47. Gao, C., et al. (2014). Horizontal gene transfer in plants, Functional and Integrative Genomics 14, 23-29
48. Soucy, S.M. et al. (2015). Horizontal gene transfer: building the web of life, Nature Reviews Genetics 16, 472-482
49. Kyndt, T, et al. (2015). The genome of cultivated sweet potato contains Agrobacterium T-DNAs with expressed genes: An example of a naturally transgenic food crop, Proceedings of the National Academy of Sciences 112, 5844-5849
50. Hopkins M (2015) DuPont, Caribou Biosciences Form Strategic Alliance, CropLife, October 8, 2015, available online: http://www.croplife.com/crop-inputs/dupont-caribou-biosciences-form-strategic-alliance/
51. Ledford, H (2016) Alternative CRISPR system could improve genome editing, Smaller enzyme may make process simpler and more exact, Nature 526, doi:10.1038/nature.2016.19114
52. Shmakov S (2015) Discovery and Functional Characterization of Diverse Class 2 CRISPR-Cas Systems, Molecular Cell 60, 385-39
53. Burnstein D et al (2016) Major bacterial lineages are essentially devoid of CRISPR-Cas viral defence systems, Nature Communications 7:10613
Download pdfFigures