Pros and cons of GM crops as a source of resistance to insect pests

Summary
Current experience of the pros and cons of using GM crops for resistance to insect pests is largely limited to one source of transgene, the proteins expressing the toxin of Bacillus thuringiensis.
Since the gene transferred, and not the method of transfer, is relevant to the topic of this paper, we can explore what is known from plants traditionally bred for insect-resistance based on similar mechanisms to those likely to be used in GM crops, i.e. on single toxins giving a high level of control.
Across a whole range of potential issues including the development of pest strains tolerant to the toxin and side-effects on natural enemies, crops with resistance mechanisms based on high concentrations of toxins compare badly with crop varieties giving partial and more broadly-based resistance.
Such partial resistance may, however, be fully effective when integrated with biological control and selective use of pesticides.
However, it must be pointed out that this is not the comparison that matters, for GM crops will not be used to replace other forms of plant resitance, but instead to replace insecticides.
In that comparison, the replacement of the spraying machine by a GM crop as the vehicle for delivering toxins has clear advantages.
Keywords
genetically modified crops, human safety, insecticide resistance, natural enemies, plant resistance, tolerance, yield loss.
Abbreviations
Bt Bacillus thuringiensis; DIMBOA 2,4 dihydroxy 7 methoxy 1,4-benzaxazin-3-one; GM genetically modified; IPM integrated pest management; USA United States of America.
Glossary
Allelochemical: A chemical produced by a living organism which, when contacted by another living organism, has deleterious effects on the latter.
Cisgene: Transgene transferred between organisms not too distantly related.
Secondary (plant) compounds: Complex chemicals made by plants but not essential to the life of the plant.
Sink (in relation to photosythesis in plants): A function in the plant creating a demand for the products of photosynthesis.
Synthesised gene: A gene with at least part of its DNA created artificially.
Transgene: Gene transferred from one organism to another by any method.
Yield drag/penalty: Reduction in crop yield caused by the diversion of photosynthesis for other purposes such as synthesis of toxins by a plant.
Introduction
The term ‘genetically modified’ (GM) has become associated with one particular type of GM, namely direct gene transfer.
Rather similar is the way ‘organic farming’ has become associated with that type of farming seen as a contrast with ‘conventional farming'.
I make this point because GM has been around since the dawn of agriculture, how else did man progress from wild grasses to productive modern cereals? ‘Traditional’ plant breeding, acceptable to the opponents of ‘GM’, is certainly GM by any definition than other that it does not involve direct gene transfer.
Focusing on the technique of gene transfer rather than the properties of the gene transferred is well expressed by the following quotation:
“We have recently advanced our knowledge of genetics to the point where we can manipulate life in a way never intended by nature. We must proceed with the utmost caution in the application of this new found knowledge”.
The fear of something ‘unnatural’ is clearly behind the opposition to GM crops. But doesn’t this exactly make my point that we should concentrate on the gene rather than the method of transfer?
Until recently one could avoid the ambiguity of GM by calling direct gene transfer ‘transgenic’, but recently the antonym ‘cisgenic’ has been proposed for the direct transfer of genes amenable to traditional breeding in an effort to find a form of GM acceptable to the ‘organic’ lobby.
While this might appear to be retreat, concentrating on the ’naturalness’ of the gene may in the end achieve acceptance that the method of transfer is not very relevant.
After all, it is not possible to limit the genes transferred by breeding to the one(s) desired; there is associated genetic baggage which, if it includes undesirable characters, may take many further cycles of breeding to eliminate; by contrast cisgenics will transfer only what is intended.
Incidentally, it is salutary to realise that the quotation above does not relate to ‘GM’ as we understand it today. It expresses the concerns of the agriculturalist Luther Burbank in 1906 when he discovered Gregor Mendel’s work with round and wrinkled pea seeds!
The first reference I can find to using transgenic methods to obtain plant resistance to insect pests is in 1979 (1), and it is clearly taking longer for ‘GM’ to be generally acceptable than applied to Gregor Mendel’s work.
‘GM’ crops have now been grown on a large scale since 1996, and the fear of ‘Frankenstein’ consequences does not appear to have any justification.
Much of the GM hectarage is planted to insect-resistant crops expressing Bacillus thuringiensis toxins (Bt); this first exploitation of ‘GM’ therefore was for resistance to insects.
Since then, genes for a number of other insect-toxic proteins (including lectins, amylase inhibitors, trypsin inhibitors, protein inhibitors, chitinases and cytokinins) have been directly transferred to crop plants, but no commercialisation has as yet followed.
If we focus on genes rather than the transfer method, the traditional plant breeding literature provides a great deal of relevant experience about the potential pros and cons of pest-resistant GM crops.
Key to this is that resistance to pests in GM crops is likely to be based on toxins, and commercial 'GM' varieties will only come to market if the genes express these toxins at a high enough level to give control equalling that given by insecticides.
So traditionally-obtained plant resistance can be directly comparable if it is based on a strong toxin. Overdosing is not limited to agrochemicals, and doing it with genes for plant resistance will have consequences not dissimilar from overdosing with insecticides.
Recently a trial of GM wheat with a synthesised gene (resynthesised from peppermint to eliminate inhibitory compounds) expressing the alarm pheromone of aphids (2) was carried out.
The aim was partly to make the point that GM crops could involve genes whose 'escape' from the trial field could not cause ecological mayhem, but such 'behavioural' and target-specific possibilities are bound to be extremely rare in comparison with toxins.
The advantages of ‘GM’ perceived by the agricultural industry are the transfer of single genes without other genetic material, that it is possible to avoid the crossing barriers that exist between unrelated organisms and that the level of expression can reach virtual immunity.
Examples from traditional plant breeding involving toxins show us that these advantages also translate to potential disadvantages (Fig. 1) and this is the theme of this article.
Safety for humans
Much of the history of the genetic modification of wild plants over past centuries, for most of that time without any understanding of Mendelian ratios, was to reduce dramatically the levels of toxins in those wild plants to make them palatable or even safe to eat.
As far as these same compounds conferred resistance to insects, so the susceptibility to pests of the crops increased and we now go back to the wild ancestors to recover these sources of resistance (Table 1 compares the levels of two toxic chemicals in crops and related non-cultivated plants).
Good examples are in the Solanaceae, where wild potatoes and tomatoes are both highly toxic to humans.
Safety for humans is paradoxically the one area of potential concern where ‘GM’ crops probably have the advantage. Because of the ‘unnatural’ angle, no one has queried the necessity for ensuring human safety.
The Bt toxin was chosen for the so-called ‘first generation’ GM crops specifically for its safety to humans stemming from differences between humans and insects at the cell molecular level.
GM crops are subject to stringent testing to ensure safety to humans before they can be marketed. In this they differ from new cultivars obtained by traditional breeding, and they have never been required to undergo similar testing before release.
A famous example of the consequences of this is the release in the USA of the potato variety ‘Lineup’, which contained elevated levels of a glycoaldehyde to confer resistance to Colorado beetle (Leptinotarsa decemlineata).
Consumers found the flavour of ‘Lineup’ distasteful, and some showed symptoms of toxicity; the new variety had to be withdrawn (3). With GM crops, the public is protected against health issues.
A further advantage is that the pest receives the toxin through the plant and not through a chemical spray hazardous to the person applying it.
Yield drag
It is hard to think of a mechanism of plant resistance to insects that does not involve some energetic cost to the plant and therefore a potential reduction in yield.
Gershenzon (4) has quantified the costs of some compounds involved in plant resistance to insects and, when expressed as per cent of photosynthesis, some costs appear dramatic.
For example, production of the triterpene papyriferic acid in birch involves a 24.5% cost, and the flavonoid apigenin (in Isocoma acradenia) a cost of 6.9%.
The photosynthetic costs of other compounds cited are lower and mostly range between 0.1 and 4.8%.
However, the real costs of producing many of such so-called ‘secondary compounds’ (or ‘allelochemicals’) are nowhere near as great as Gershenzon and many other authors including myself (3) have suggested in the past.
Firstly photosynthesis is ongoing whereas production of allelochemicals is not, and secondly radiation is mostly sufficient for photosynthesis to be sink rather than source limited – i.e. the cost of allelochemical production can be compensated for by a rise in photosynthetic rate (4).
Thus, referring back to Table 1, the high glucosinolate levels in Sisymbrium officinale can be achieved in only 7.5 minutes of photosynthesis, and the levels of 2-tridecanone in wild tomato take less than one hour (4).
Such short times would be typical for annual crops rather than for perennials which make lifetime investments in much more energy-expensive defence compounds such as lignin and tannins that may accumulate to 20% of dry matter.
It is therefore not surprising that yield penalties of plant resistance have been hard to demonstrate, including for the quite complex molecules of the Bt toxins.
Any potential for yield loss will of course be minimised if partial plant resistance is combined with other pest control methods (Integrated Pest Management or IPM) in contrast with the sole reliance on an allelochemical as is characteristic of GM.
However, the latter resistance does have one advantage. In the modification process it may be possible to add promoters which result in the transgenically transferred toxin only being expressed if induced by pest attack. Therefore there can be no yield penalty of the resistance in the absence of the pest.
Tolerant pest strains
It is a familiar scenario that continued use of an effective insecticide eventually leads to it losing that effectiveness because pest strains in the population are selected that are tolerant (resistant).
Such ‘breakdown of control’ is also very familiar to plant pathologists using resistant varieties to control plant disease organisms such as rust fungi in cereals.
With plant resistance, such breakdown is most frequently associated with single major genes for resistance, usually providing only what is known as ‘race-specific resistance’. It is therefore only to be expected that the high selection pressure on a pest imposed by a single effective transgenically transferred toxin will similarly result in ‘breakdown’.
In a study of 77 reports from eight countries in five continents (6), more than 50% resistance to Bt crops was found in five out of 13 pest species in 2-011, compared with only one in 2005. In the laboratory, it has been possible to select one of the cotton bollworms (Pectinophora gossypiella) for resistance to Bt cotton in only 10 generations.
The widespread expectation of the breakdown of GM pest resistance has led to attempts to checkmate this development by requiring farmers to maintain areas of non-GM cotton to act as ‘refugia’ where moths with some resistance to Bt can mate with susceptible ones.
With scientific opinion originally divided on the merits of this approach, this programme was itself an experiment, but does seem to have worked pretty well where the strategy has been implemented.
Whereas growing Bt cotton with the strategy in Arizona has shown sustained susceptibility in pink bollworm (Pectinophora gossypiella), control failures without the strategy have been reported in China and India (7).
The danger of breakdown of resistance with GM crops stems from the high selection pressure the expression of the gene puts on the pest population and so applies equally to traditional plant resistance based on highly effective allelochemicals – the mode of transfer of the gene is irrelevant!
An example of such breakdown in traditional plant resistance is that of monogenic resistance in rice to brown planthopper (Nilaparvata lugens) developed at the International Rice Research Institute in the Philippines.
One new variety (IR26) was particularly resistant to the planthopper, but was defeated within only 2-3 years by the appearance of a strain that was tolerant to the resistance (8).
By contrast, many examples of plant resistance which are not so strong, are multigenic and/or based on non-allelochemical methods such as tissue hardness, have lasted for many years.
As an example, the rootstocks of the vines of old French communion wines, taken to the USA by early settlers from Europe, were grafted in the early 19th century with scions of vine varieties then current in Europe to deal with the destruction being wrought by an aphid (Phylloxera).
The resistance this provided is still largely effective today after 200 years, though a tolerant Phylloxera did appear in a small area in Germany in 1994 (9).
Indeed, in a review of the literature in 1996 (3) I was only able to find 16 pest species in which resistance-breaking strains had been identified; moreover, several were examples of selection in the laboratory rather than in the field.
As mentioned earlier, traditional breeding contrasts with GM in resulting in the transfer of a genetic package which includes the desired gene amongst others, and so the resistance may not be monogenic if other unknown genes also confer some resistance.
Thus resistance to aphids in cereals, thought to be monogenically based on just one compound (the 1,4-benzaxazin-3-one glycoside DIMBOA), involves other resistance mechanisms also (10).
Problem trading
One of the advantages the industry claims for GM crops with plant resistance to pests is that it is environmentally preferable to the heavy use of insecticides it replaces.
Again, there is nothing special here about GM; the advantage could equally be claimed for traditionally bred resistant varieties.
However, an insecticide usually controls more pest species on the crop than the one target organism, and so another pest may build up in numbers and become a new problem once insecticide pressure on the crop is relaxed. This has actually happened in Bt cotton with leafhoppers emerging from little to major economic importance (11).
Damage to beneficial insects
Whatever the mechanism of transfer of a gene conferring high expression of a toxin, natural enemies will lose their food supply unless the crop also hosts alternative prey not affected by the toxin.
In relation to implementing IPM on crops, there is of course no mileage in trying to combine different methods of pest control if any one of the methods is effective on its own.
Toxins in plants, whatever their origin or method of triansfer, are also likely to affect natural enemies directly if they eat poisoned prey on the plants.
There are plenty of examples where chemical defence, whether in wild or cultivated plants, also proves toxic to predators and/or parasitoids; some of these are listed by van Emden (12).
Allelochemicals are important in soybean varieties bred for resistance to pests and such varieties are known to affect natural enemies adversely (13).
One interesting case – sadly probably a rare exception – where high levels of allelochemicals are actually less damaging than lower levels is that of the aphid-resistance factor DIMBOA in wheat.
At high levels, the effect on aphids is not acute toxicity, but to deter their feeding. The ladybird predator Eriopis connexa is therefore not as badly affected as it is when eating aphids poisoned on cultivars with lower DIMBOA levels (14).
The few toxins so far exploited for transgenic transfer to crops have of course been tested for any deleterious side effects on natural enemies of feeding on prey on such plants.
Much of this work relates to Bt, and a review in 2004 (15) of some 25 laboratory and field studies concluded Bt crops would present few problems, and this has been confirmed in more recent studies (e.g. 16, 17).
One rather inconclusive laboratory study did suggest high mortality of lacewing larvae when fed caterpillars reared on Bt maize (18). In fact, Bt is rather selective in which taxa it affects, and honey bees are not affected (19, 20).
Also, there appears to be considerable dilution through the trophic levels (21). The concentration of 21.7 µg g-1 fresh weight of a Bt toxin (Cry3Bb 1) in maize reduced to 5.6 µg g-1 in mites feeding on the maize, and was reduced further to about 4.5 and only 1.4 µg g-1 respectively in larvae and adults of a predatory staphylinid beetle.
Other transgenes proposed for pest resistance seem to pose greater problems in relation to beneficials. The ladybird Adalia bipunctata fed aphids reared on the leaves of potatoes expressing the snowdrop lectin showed no acute symptoms, but female longevity was reduced by half and fecundity by 20-40% (22).
Lectins are also toxic to bees (20). Amylase and protease inhibitors proposed for GM legumes against bruchid beetles have been tested against their parasitoids with conflicting results (23, 24); harmful affects cannot be discounted.
The high efficacy of pest resistance in GM crops makes it difficult if not impossible to exploit the often positive interaction between plant resistance and biological control, whether with indigenous natural enemies present in the agroecosystem or augmented artificially (e.g. by release).
These interactions include highly specific ones such as the switch of Cryptolaemus ladybirds from nectar-feeding to carnivory on cotton varieties on which plant breeding has eliminated the extra-floral nectaries on the leaves (25) and the improved predation of aphids by adult ladybirds on the so-called ‘leafless’ pea varieties with a profusion of leaf-substituting tendrils.
These substitute for leaves and afford ladybirds a goodgrip, whereas they frequently fall off the smooth waxy leaves of normal varieties (26).
However, positive synergism between plant resistance and biological control is a widespread and much more general phenomenon (3) than the two examples above might suggest , though it cannot be taken for granted (e.g. the examples based on toxins cited above).
Positive synergism may arise from numerical relationships, i.e. the predator/parasitoid prey ratio is higher on partially resistant than on more pest-susceptible varieties.
This occurs particularly when the natural enemies use host plant cues (especially odours) in searching for prey from a distance.
Parasitoids often emerge with a preference for the odour of the host plant (species and even variety) on which they themselves developed (e.g. parasitoids of aphids – (27)).
Furthermore the plant odour changes where sucking insects have probed, which enables the parasitoids to home in on the location of even a few prey (as is only to be expected on resistant plants).
Thus biological control in Africa of the cassava mealybug by its parasitoid Epidinocarsis lopezi is primarily achieved when the prey are already at very low numbers in the dry season.
More usually, perhaps, the positive synergism between partial plant resistance and biological control depends on functional responses, i.e. there is a greater impact per individual predator on resistant than susceptible varieties.
The common phenomena (12) appear to be:
1) Prey on resistant plants tend to be smaller – predators will therefore consume more before they are satiated.
2) Smaller prey are also less likely to escape by running away or kicking at the natural enemy.
3) Disturbance by predators/parasitoids may cause the prey to fall from the plant in greater numbers on resistant than on susceptible plants (eg. 28).
4) Higher pest densities on the leaves of susceptible plants may leave more wax, faeces, honeydew etc. which may impede the movement of natural enemies or cause them to devote searching time to cleaning.
A simplistic but nonetheless adequate approach to measuring synergism is that of multiplying the survival coefficients of the synergising components.
If, in a given time interval, biological control reduces a pest population by 10% (i.e. survival coefficient = 0.9), the population on a plant with 20% resistance (i.e. survival coefficient = 0.8) should be 0.9 x .08 = 0.72 = 72% of that on the susceptible variety without biological control. Fig. 2 shows this calculation (expressed as percentages) applied to a range of data from the literature (12).
Thus there is a potential in IPM for exploiting positive synergism between plant resistance and biological control which is unlikely to be available with GM crops or with traditionally bred resistant varieties expressing similarly high levels of allelochemicals.
Resistance to insecticides The toxicity of insecticides, whether to insects or humans, is measured as dose of active ingredient per unit body weight.
Therefore, as insects on resistant plants are usually smaller than on susceptible plants, it would be surprising if the same dose of chemical did not kill a higher proportion.
There are many examples illustrating this phenomenon (e.g. Fig, 3), but the effect is usually greater than predicted from the reduction in body weight alone; a greater ‘physiological’ sensitivity also seems to be involved.
In fact, Fig. 3 suggests that the concentration of pesticide applied to susceptible plants to kill 50% of the pests (LC50) could be reduced by more than one-third on the compared resistant plants (12).
However, there are equally examples where pests on resistant varieties have increased resistance to insecticides.
The explanation appears to be that exposure to allelochemicals in plants induces an increase in enzymes which can detoxify these chemicals, but also other toxins (including insecticides).
This effect has been shown convincingly with the bollworm Heliothis virescens on cotton varieties with a high gossypol content (29) and by measuring the activity of one such detoxifying enzyme (·-naphthyl esterase) in armyworms (Spodoptera frugiperda) fed on eight crop plants known to contain toxic allelochemicals (30).
Finally, there was a reduction in mortality from the insecticide carbaryl on resistant (with high levels of 2- tridecanone) compared with susceptible tomato plants (31), with almost identical mortalities when the varieties were replaced respectively with artificial diet + 2-tridecanone and standard artificial diet (Fig. 4).
Similarly, addition of the glucosinolate sinigrin to an artificial diet increased the tolerance of diamond back moth (Plutella xylostella) progressively between 0 and 0.125 μmol sinigrin g - 1 diet (32).
Thus on crop varieties with high levels of allelochemicals, whether transgenically or traditionally “bred”, the pests may show resistance to insecticides should these have to be used as, for example, if individuals tolerant to the plant resistance appear in the population.
However, there is a further disadvantage of such varieties in relation to insecticides. It was pointed out above that, on many pest-resistant varieties traditionally bred, a lower dose of pesticide will give the same level of control as on a susceptible variety.
Those lower doses will, of course, achieve a reduction in the kill of natural enemies – immediately the selectivity of the pesticide application in favour of the natural enemies will improve. This is pure IPM!
However, there is more to it than that, since this increase in selectivity will be far greater than one might at first envisage. Over 30 years ago, a fundamental difference in the response to insecticides of herbivores and carnivores was identified (33).
The former have evolved to cope with allelochemicals in the plants they feed on by producing detoxifying enzymes. Genetically, individuals will differ in their ability to do this.
This will result in a large difference in the insecticide concentration required to kill the most resistant compared with the most susceptible individual (Fig. 5A). By contrast, carnivores, feeding on prey which has largely dealt with any toxins in the plant, have less need for any armoury of detoxifying enzymes, and so there will be less spread in the pesticide concentration needed to kill different individuals (Fig. 5A).
Fig. 5B shows how the selectivity window for the natural enemy increases dramatically as pesticide concentration is reduced when only the response of the herbivore is affected.
However, exploiting this phenomenon will be difficult in those countries which legislate against deviating from the manufacturer’s recommended dose.
The theory behind Fig. 6 has been tested in the laboratory (34), using the cereal aphid Metopolophium dirhodum as the pest with the parasitoid Aphidius rhopalosiphi and larvae of the ladybird predator Coccinells septempunctata as natural enemies.
Table 2 gives the doses for the pesticide malathion required to kill 50% and 90% of the respective insects on two wheat cultivars, the aphid-susceptible Maris Huntsman and the only partially resistant Rapier.
Not only were the doses for the carnivores not reduced by the plant resistance, they were actually somewhat increased, particularly for the ladybird.
Conclusions
Genetic engineering of plant resistance to insects has relied on the direct transfer of single genes for the production of allelochemicals deleterious to plant-feeding pests.to the point of virtual immunity.
It is not difficult to find potential disadvantages of this approach, but it must be remembered that insect-resistant GM crops are only commercial because they control serious pest problems effectively.
Therefore it is nonsense, as the media and public so often do – to compare them with doing nothing in the way of pest control – for that is hardly an option.
GM crops do have disadvantages (Table 3) when compared with plant resistance obtained by traditional plant breeding, especially since the lower control efficacy usually provided by these can often synergise with biological control and/or limited use of pesticides to give grower-acceptable pest control.
How can any synergism occur if one of the methods involved gives total control on its own? Of 13 mechanisms of plant resistance developed by traditional plant breeding (34), only three involve allelochemicals.
Some progress with GM is being made in making the expression of the allelochemical dependent on pest attack occurring, and in transferring more than a single gene for resistance, but the single gene usually giving constitutive protection is still the norm.
However, in nearly every case, a pest-resistant GM crop will be used to replace (usually heavy) insecticide applications.
This is therefore the most important comparison, and GM crops clearly have major advantages (Table 3), particularly in terms of human safety, in selectivity in favour of natural enemies and less environmental contamination.
In general, natural chemical compounds are more easily and rapidly broken down to harmless compounds in the environment than are synthetic ones.
Perhaps pest-resistant GM crops would get a better press and be more readily accepted by the public if they were viewed as an alternative to the spraying machine as a way of delivering insecticide!
References
1. Levin, B R (1979) Problems and promise in genetic engineering in its potential applications to insect management. In: Genetics in relation to pest management (eds M A Hoy & J M McElvey, Jr), New York, Rockefeller Foundation, pp. 170-175.
2. Bruce, T J A, Smart, L E, Aradottir, G J, Martin, J L et al. (2011) Trangenic wheat emitting aphid alarm pheromone. (E)-beta-farnesene, Aspects of Applied Biology, 110, 112.
3. van Emden, H F (1996) Host-plant resistance to insect pests. In: Techniques for reducing pesticide use (ed. D Pimentel), Chichester, Wiley, pp. 130-152.
4. Gershenzon, J (1994) The cost of plant chemical defense against herbivory. A biochemical perspective. In: Insect-plant interactions, vol. 5 (ed. E A Bernays), Boca Raton, CRC Press, pp. 173-205.
5. Foyer, C H, Noctor, G. & van Emden, H F. (2007) An evaluation of the costs of making specific secondary metabolites; is the yield penalty incurred by host plant resistance to insects due to competition for resources? International Journal of Pest Management, 53, 175-182.
6. AFP (2013) ‘More pests resistant to GM crops’: a study <http://www.google.com/hostednews/afp/article/ALeqM5gkQ3lN4hx0r59smxAQgaWzlWKaVg> accessed 10 03 2014.
7. Tabashnik, B E, Morin, S, Unnithan, G C, Yelich, A.J. et al. (2012) Sustained susceptibility of pink bollworm to Bt cotton in the United States (special issue on insect resistance). GM Crops, 3, 194-200.
8. Panda, N & Khush, G S (1995) Host-plant resistance to insects. Wallingford, CAB International.
9. Anon (1994) Die Rükkehr der Reblaus. Profil, October 1994, 11.
10. Gallun, R L (1977) The genetic basis of hessian fly epidemics. In: The genetic basis of epidemics in agriculture (ed. P R Day). Annals of the New York Academy of Sciences, 287, 1-400.
11. Steinkraus, D C, Young, S Y, Gouge, D H] & Leland, J E. (2007). Microbial insecticide application and evaluation: Cotton. In: Field manual of techniques in invertebrate pathology, 2nd edn. (eds L A Lacey & H K Kaya), Dordrecht, Springer, Section VII-6.
12. van Emden, H F (1999) Transgenic host plant resistance to insects – some reservations. Annals of the Entomological Society of America, 92, 788-797.
13. Boethel, D J (1999) Assessment of soybean germplasm for multiple insect resistance. In: Global plant genetic resources for insect-resistant crops (eds S L Clement & S S Quisenberry), Boca Raton, CRC Press, pp. 101-129.
14. Martos, A, Givovich, A & Niemeyer, H (1992) Effect of DIMBOA, an aphid resistance factor in wheat, on the aphid predator Eriopsis connexa Germar (Col.: Coccinellidae). Journal of Chemical Ecology, 18, 469-479.
15. Schuler, T H (2004) GM crops: good or bad for natural enemies? In: GM crops – ecological dimensions (eds H F van Emden & A J Gray). Aspects of Applied Biology, 74, 81-90.
16. Svobodova, Z, Habustova, O, Hussein, H M, Puza, V & Sehnal, F (2012) Impact of genetically modified maize expressing Cry3Bb1 on non-target arthropods: first year results of a field study. IOBC/WPRS Bulletin, 73, 1-8.
17. Albajes, R, Lumbierres, B. Madeira, F, Comas, C et al. (2013) Field trials for assessing risks of GM maize on non-target arthropods in Europe: the Spanish experience. IOBC/WPRS Bulletin, 97,1-8.
18. Hilbeck, A, Baumgartner, M, Fried, P M. & Bigler, F (1998). Effects of transgenic Bacillus thuringiensis corn-fed prey on mortality and development time of immature Chrysoperla carnea (Neuroptera: Chrysopidae). Environmental Entomology, 27, 1-8.
19. Malone, L A, Todd, J H, Burgess, E P J & Philip, B A (2004) Will GM crops expressing insecticidal proteins harm honey bees? In: GM crops – ecological dimensions (eds H F van Emden & A J Gray). Aspects of Applied Biology, 74, 114-118
20. Hendriksma, H P, Hartel, S, Babendreier, D, Ohe, W & von der Steffan-Dewenter, I (2012) Effects of multiple Bt proteins and GNA lectin on in vitro-reared honey bee larvae. Apidologie, 43, 549-560.
21. Garcia, M, Ortego, F, Castanera, P & Farinos, G (2012) Assessment of prey-mediated effects of the coleopteran-specific toxin Cry3Bb1 on the generalist predator Atheta coriaria (Coleoptera: Staphylinidae). Bulletin of Entomological Research, 102, 293-302.
22. Birch, A N E, Geoghegan, I E, Majerus, W E N, McNicol, J W et al. (1988). Tri-trophic interactions involving pest aphids, predatory 2-spot ladybirds and transgenic potatoes expressing snowdrop lectin for aphid resistance. Molecular Breeding, 5, 75-83.
23. Alvarez-Alfageme, F, Luthi, C & Romeis, J (2012) Characterization of digestive enzymes of bruchid parasitoids-initial steps for environmental risk assessment of genetically modified legumes. PLoS ONE, 7(5), e36862.
24. Luthi, C, Alvarez-Alfageme, F & Romeis, J (2013) Impact of alpha AI-1 expressed in genetically modified cowpea on Zabrotes subfasciatus (Coleoptera: Chrysomelidae) and its parasitoid, Dinarmus basalis (Hymenoptera: Pteromalidae). PLoS ONE, 8(6), e677857.
25. Adjei Maafo, I. K (1980) Effects of nectariless cotton trait on insect pests, parasites and predators with special reference to the effects on the reproductive characters of Heliothis spp. PhD thesis, University of Queensland, Australia.
26. Kareiva, P & Sahakian, R (1990) Tritrophic effects of a simple architectural mutation in pea plants. Nature, 345, 433-434.
27. Wickremasinghe, M G V & van Emden, H F (1992) Reactions of female parasitoids, particularly Aphidius rhopalopsiphi, to volatile chemical cues from the host plants of theiraphid prey. Physiological Entomology, 17: 291 304.
28. Gowling, G R & van Emden, H F (1994) Falling aphids enhance impact of biological control by parasitoids on partially aphid resistant plant varieties. Annals of Applied Biology, 125, 233 242.
29. Shaver, T N & Wolfenbarger, D A (1976) Gossypol: influence on toxicity of three insecticides to tobacco budworm. Environmental Entomology, 5, 192-194.
30. Yu, S J & Hsu, E L (1985) Induction of hydrolases by allelochemicals and host plants in fall armyworm (Lepidoptera: Noctuidae) larvae. Environmental Entomology, 14, 512-515.
31. Kennedy, G G (1984) 2-tridecanone, tomatoes and Heliothis zea: potential incompatibility of plant antibiosis with insecticidal control. Entomologia Experimentalis et Applicata, 35, 305-311.
32. Hariprasad, K V & van Emden, H F (2014) Effect of partial plant resistance in brassicas on tolerance of diamondback moth (Plutella xylostella) larvae to cypermethrin. International Journal of Pest Management, 60 (in press).
33. Plapp, F W Jr (1981) Ways and means of avoiding or ameliorating resistance to insecticides. Proceedings of Symposia at thr 9th International Congress of Plant Protection, Washington D.C., 1979, 1, 244-249.
34. Tilahun, D A & van Emden, H. (1997) The susceptibility of rose grain aphid (Homoptera: Aphididae) and its parasitoid (Hymenoptera: Aphidiidae) and predator (Coleoptera: Coccinellidae) to malathion on aphid susceptible and resistant wheat cultivars. Annales de la 4e ANPP Conférence Internationale sur les Ravageurs en Agriculture, Montpellier, 1997, 1137-1148.
35. van Emden, H F & Service, M W (2004) Pest and Vector Control. Cambridge, Cambridge University Press.
Download pdf
Figures
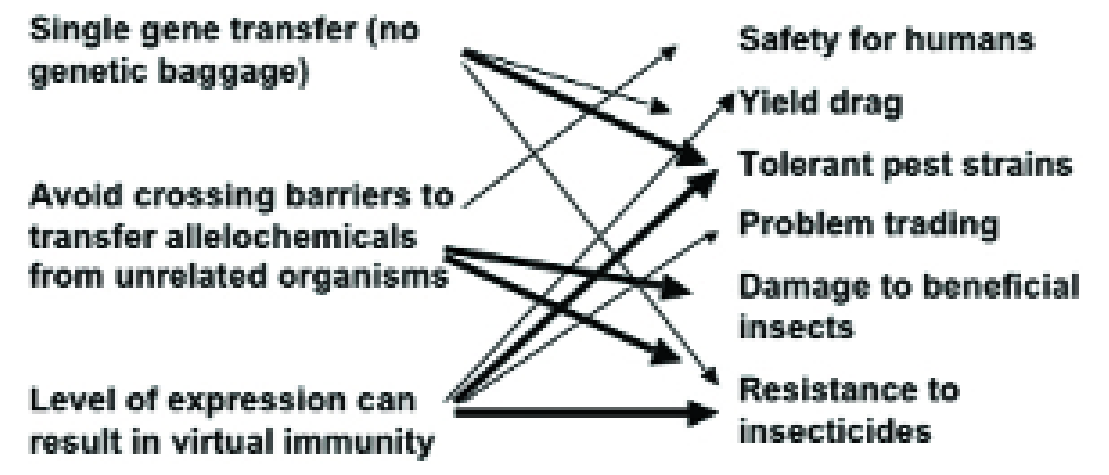
Figure 1. How the perceived advantages (left) of transgenic host plant resistance to insects have the potential for disadvantageous side effects (right)

Table 1. Quantities of some allelochemicals in cultivated (C) and wild (W) tomatoes and brassicas/
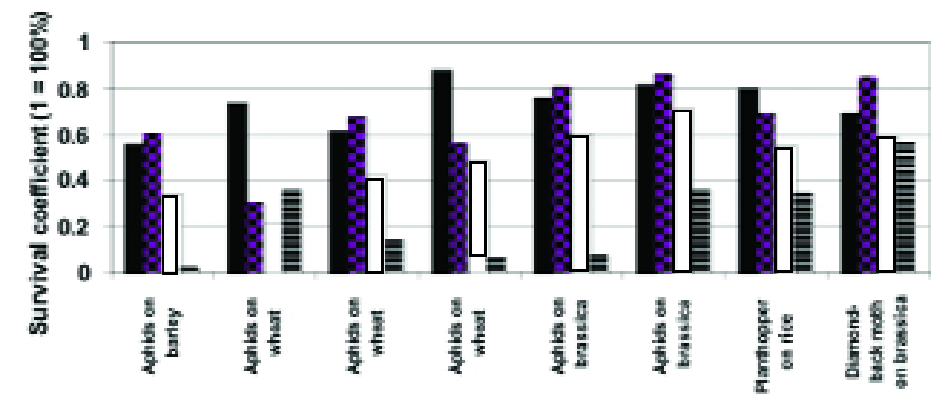
Figure 2. Some examples of the outcome of combining host plant resistance to insects with biological control (histograms show the proportion of insects “surviving” the control measures). Histograms in each block from left to right: plant resistance alone (black), biological control alone (checkerboard), prediction of control given by the combination (white), actual outcome (striped).
Note: the second block of histograms form the left illustrate negative interaction and the example of diamond-back moth (far right) show simple additivity of the two control measures. More detail of the pest species and sources of the data can be found in (12).
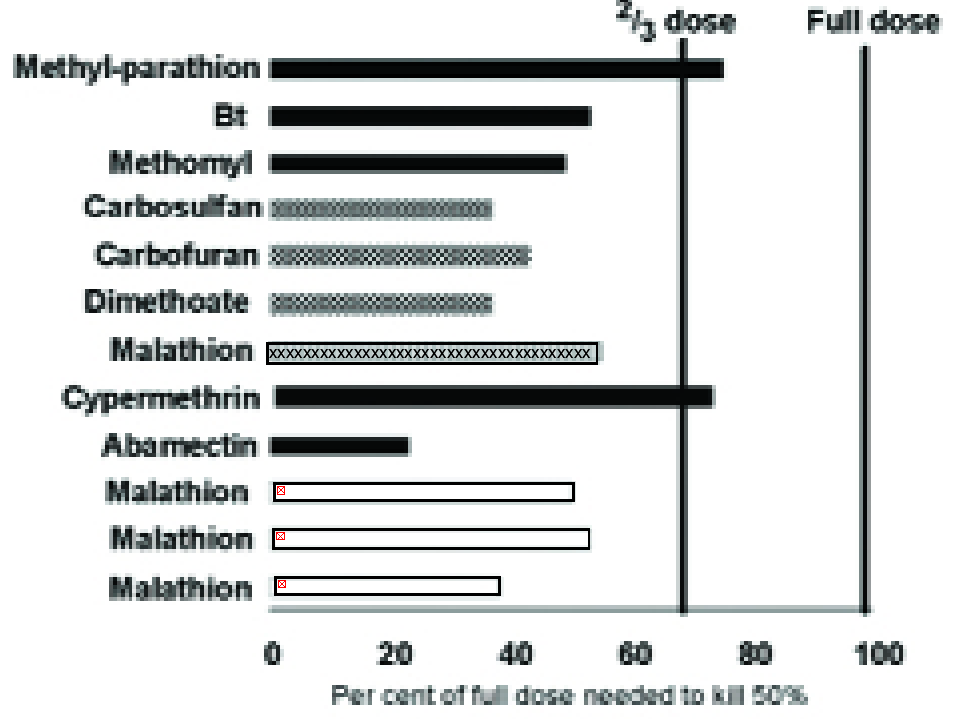
Figure 3. The concentration of different insecticides (listed on left) needed on a partially resistant crop variety to achieve 50% mortality expressed as a percentage of the concentration needed on a susceptible variety. Key to pests: hoppers (checkerboard), aphids (white), Lepidoptera (black), Coleoptera (x striped). More detail of the pest species and sources of the data can be found in (12).
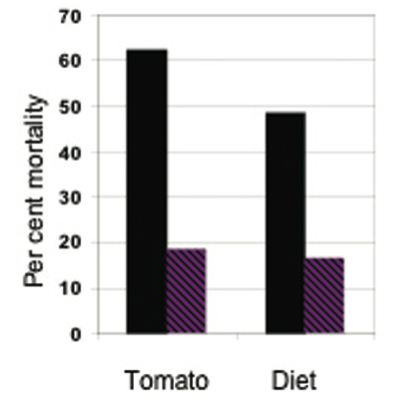
Figure 4. Effect of the allelochemical 2-tridecanone on mortality of bollworms (Helicoverpa zea) from the insecticide carbaryl. Left, comparison between a susceptible (black) and a resistant (striped) variety of tomato; right, comparison between two artificial diets – one without (black) and the other with (striped) added 2-tridecanone (data from (31).
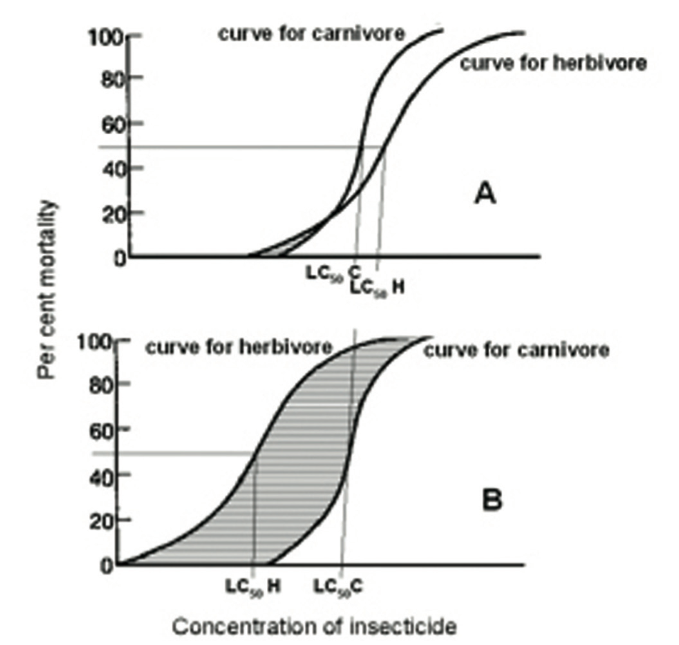
Figure 5. Theoretical insecticide concentration/insect mortality response curves for a pest (herbivore) and a natural enemy (carnivore) on a susceptible crop variety (A) and on one where 50% mortality of the pest (LC50H) is obtained at only 2/3 of the concentration needed on the susceptible variety (B). LC50C, concentration needed to obtain 50% mortality of a natural enemy; striped area, the ‘selectivity window’ (where the proportion of the pest killed is higher than that of the natural enemy).
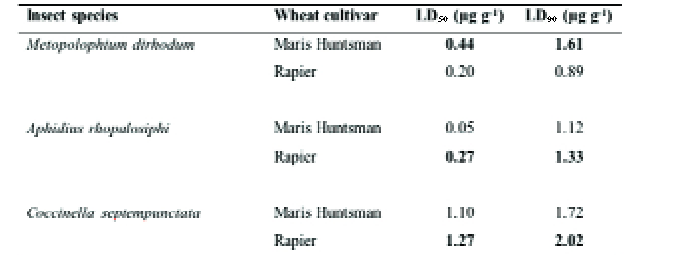
Table 2. Laboratory estimates of the toxicity of the insecticide malathion to the aphid Metopolophium dirhodum and to two of its natural enemies (the parasitoid Aphidius rhopalosiphi and the larva of the coccinellid Coccinella septempunctata) when reared on the aphid-susceptible wheat cv. Maris Huntsman and the partially resistant cv. Rapier. LD50 and LD90, the dose needed to kill respectively 50 and 90% of the insects. In each pair of figures, the one showing greater tolerance to malathion is emboldened.
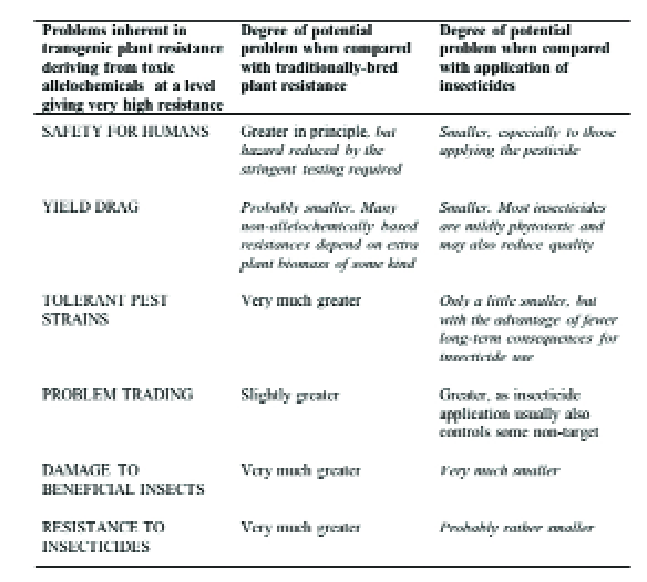
Table 3. Comparison of transgenic host plant resistance to insects with plant resistance obtained by traditional plant breeding and with the use of insecticide.
Comparisons favourable to transgenic resistance are italicised.

