Oils and fatty acids essential for vertebrate health – e.g. fish and Man

Summary
This paper provides explanations both of why essential fatty acids are essential and of biochemical terms which are widely used by the general press.
These explanations should aid an appreciation of two of our papers in this issue especially that by Haslam et al. Vegetable oils are extracted mostly from seeds of maize, soyabean, sunflower, linseed, rape, olive, and palm.
These oils provide over twice as much net energy/kg compared with starch and so their consumption can contribute to obesity with its adverse effects on health.
They are a source of two essential dietary nutrients in the form of the polyunsaturated fatty acids (PUFA), C18:2 6 linoleic acid and C18:3, 3, a-linolenic acid.
Olive oil is also a rich source of C18:1, 9, oleic acid, a monounsaturated fatty acid (MUFA), which may contribute to its health benefits. Long chain omega-3 fatty acids, C20:5, 3, eicosapentaenoic acid (EPA), and C22:6, 3, docosahexaenoic acid (DHA) are also essential in the diet of vertebrates, but the only major primary source of them is oceanic mono-cellular organisms, phytoplanktons and algae, the foodstuff of oceanic animal life.
Thus, production of these oils from a crop which can be grown in temperate climates will be an essential source for fish farming, where their current source from wild fish oils, is becoming scarce. EPA and DHA consumption beneficially influence risk factors of cardio-vascular disease (CVD) when they replace saturated fats in the diet.
They are more potent than are a-linolenic acid and linoleic acid, or than oleic acid. All these unsaturated fatty acids are protective against CVD, in comparison to saturated fatty acids, e.g. palmitic acid (C16:0). Nevertheless, palm oil is rich in both palmitic acid and oleic acid (Table 1).
Olive oil contains a larger proportion of oleic acid and less palmitic acid than does palm oil, and is considered to be protective. Nevertheless, unsaturated fats are prone to oxidation and rancidity, whereas saturated fats are relatively stable and the hydrogenation of unsaturated vegetable oils during the production of margarine leads to cis—trans isomerisation of unsaturated fats.
The trans-isomers have been shown to raise plasma LDL cholesterol levels and to pose a risk for CVD. Hence, legislation has been introduced in the USA, where trans-fat levels must be declared on the labels of appropriate products in the USA.
However, compared with the relationship of fatty acids with the risk factors of CVD, the correlation of all these risk factors with the occurrence of CVD is less well secure, for the obvious reason that individuals do not eat nutrients, but mixed diets; so there is confounding of factors on the response to the dietary environment of individuals. Some clarification of this situation is possible in population studies.
Key words
fats, lipids, risk factors, CVD, GM, definitions
Abbreviations
CVD, cardiovascular disease; GRAS, generally recognized as safe; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids.
Definitions – what are fats?
Lipids
Lipids are a group of naturally occurring compounds that include fats, waxes, sterols, including cholesterol, fat-soluble vitamins (such as vitamins A, D, E, and K), monoglycerides, diglycerides, triglycerides, phospholipids, and others.
The main biological functions of lipids include storing energy, signalling and acting as structural components of cell membranes.
Lipids have applications in the cosmetic and food industries as well as in nanotechnology. Although the term lipid is sometimes used as a synonym for fats, fats are a subgroup of lipids called triglycerides or triacylglycerols.
Triglycerides (Triacylglycrols )
Triglycerides are the main constituents of vegetable oil (typically containing more unsaturated fatty acids) and animal fats (typically with more saturated fatty acids), see Figure 1 for a typical structure.
Figure 2 shows four different fatty acids. Stearic acid is completely saturated, i.e. it has single bonds (-CH2-CH2-) between the carbon atoms in its chain.
Saturated fatty acids are “saturated” with hydrogen – all available places where hydrogen atoms could be bonded to carbon atoms are occupied i.e. each carbon has two H atoms attached to it, whereas the omega (end of the C chain) will have three i.e. a methyl group.
Unsaturated compounds have double bonds (-CH=CH-) between carbon atoms, reducing the number of places where hydrogen atoms can bond to carbon atoms to one H atom per C atom, as in oleic acid and in a-linolenic acid (Fig. 2).
These carbon bonds are under stress and thus open to oxidation, or to rotation.
The fatty acid bonds to the glycerol through its acyl (carboxyl) group in forming a fat. Unsaturated fats have a lower melting point and are more likely to be liquid at room temperature.
Saturated fats have a higher melting point and are more likely to be solid at room temperature. Melting point is also determined by C chain length of the fatty acids shorter chains have a lower melting point. Most natural fats contain a complex mixture of individual triglycerides. Because of this, they melt over a broad range of temperatures.
Polyunsaturated fatty acids act as valuable structural components in cell membranes, whereas saturated fats, not used as energy sources, tend to accumulate in the liver, viscera and subcutenaeous fat depots and are a component of arterial plaques.
Fatty acids
These are chain-like molecules, the carboxylic acid (-COOH) end of which is considered the beginning of the chain, thus “alpha” with the methyl (CH3) end, as the “tail” of the chain, thus “omega.”
Nevertheless, the way in which a fatty acid is named is determined by the location of the first double bond, frequently counted from the methyl end, that is, the omega ( -) or the n- end.
The chain lengths of the fatty acids in naturally occurring triglycerides vary, but most contain 16, 18, or 20 carbon atoms. Natural fatty acids found in plants and animals are typically composed of only even numbers of carbon atoms.
Bacteria, however, possess the ability to synthesise odd- and branched-chain fatty acids.
As a result, ruminant animal fat contains some odd-numbered fatty acids, such as 15, owing to the action of bacteria in the rumen. (see Table 1)
A short notation is used to define those of each type of fatty acid n.b. see under Transisomers, below, for an explanation of the term “cis”:
C18:0 denotes an acid with 18 C atoms in its chain, all of which are saturated with H atoms, a SFA.
C18:1, 9 (also known as C18:1n-9), oleic acid, a MUFA, denotes a fatty acid with 18 C atoms in its chain, but it has one unsaturated cis-bond, which is 9 C atoms from the omega ( or n) end, i.e. the methyl-group, of the chain.
C18:2, 6 (also C18:2n-6), linoleic acid, a PUFA, denotes a fatty acid with 18 C atoms in its chain, but it has two unsaturated cis-bonds, the first of which is 6 C atoms from the end of the chain, whereas the second double bond is 9 C atoms from the end of the chain.
This is a polyunsaturated fatty acid (PUFA). It is one of the essential fatty acids, so called because they are necessary for health, and they cannot be produced adequately within the human body. They must be acquired through diet.). All PUFAs are dietary essential in humans.
C20:5, 3 (also C20:5n-3), EPA, a PUFA with 20 C atoms in its chain with five unsaturated cis-bonds, the first of which is 3 C atoms from the end of the chain, whereas the other double bonds are 6, 9, 12 &15 C atoms from the end of the chain.
C22:6, 3 (also C22:6N-3), DHA, a PUFA with 22 C atoms in its chain, but with six unsaturated cis-bonds, the first of which is 3 C atoms from the end of the chain, whereas the other double bonds are 6, 9, 12 ,15 & 18 C atoms from the end of the chain. This is
another PUFA.
C18:3, 3, a-linolenic acid (ALA), an n-3 PUFA with an 18-carbon chain and three cis double bonds. The first double bond is located at the third carbon from the methyl end of the fatty acid chain, known as the n end- the other two are located at carbons 6 and 9.
Thus, a-linolenic acid is a polyunsaturated n-3 (omega-3) fatty acid. ALA is found in seeds: chia, flaxseed, nuts (notably walnuts), and in many common vegetable oils. In terms of its structure, it is also named from the alpha-end as all-cis-9,12,15-octadecatrienoic acid. Its isomer is gamma linoleic acid GLA is 18:3 (n-6).
n.b. I used the term cis. It is unfortunately important to understand this term, as legislation is in the process for the declaration of the cis-trans composition of fats in food products (see Trans-Isomers, below).
N-3, Omega, 3
Terrestrial plants can be rich in 18C PUFA such as linoleic acid (18:2 6) and a-linolenic acid (ALA, 18:3 3), but the biologically most active omega-3 fatty acids in fish are the long-chain polyunsaturated 3 fatty acids primarily EPA (20 carbons and 5 double bonds) and DHA (22 carbons and 6 double bonds) (Fig. 3).
Some freshwater and salmonid species of fish can produce EPA and then from EPA, the more crucial, DHA, but most marine fish are unable to do so in adequate quantities from the shorter-chain omega-3 fatty acid ALA (18 carbons and 3 double bonds) provided in dietary plant sources.
The ability of vertebrates to make these longer-chain omega-3 fatty acids from ALA may be impaired even more by aging, and is generally considered not to exceed 5%, so this ability is inadequate to meet their dietary requirement.
It may be concluded that vertebrates, including fish, cannot adequately synthesise PUFAs (i.e. fatty acids with more than one double unsaturated bond) because they lack the enzymes required for their production from monounsaturated fatty acids.
Trans-isomers (see Fig. 4)
An unwelcome side effect occurs during the partial hydrogenation of the unsaturated fat when some of the cis double bonds are converted into trans double bonds by an isomerization reaction with the catalyst used for the hydrogenation during production to raise the melting point of products such as margarine.
Cis and trans forms are known as geometric isomers of fatty acids. They are structurally identical except for the arrangement of the double bond, and so the location of the two H atoms in relation to the two carbons is changed.
Most natural fatty acids have a cis-configuration i.e. vegetable sources.
The fatty acids in Figs 2 and 3 are all indicated as cis-isomers. Fig. 4 indicates the change in the bond during trans formation – a structural arrangement possessing a lower energy.
There is pressure on legislators to place a legal limit on trans-isomers of unsaturated fatty acids, especially in hydrogenated vegetable fats. In 2013, the United States Food and Drug Administration (FDA) issued a preliminary determination that partially hydrogenated oils (which contain trans fats) are not “generally recognized as safe”.
On 16 June 2015, the FDA in the USA set a three-year time limit for their removal from all processed food. Trans fats levels can be reduced or eliminated using saturated fats!1.
High intake of trans fatty acids can lead to human health problems as it contributes to obesity, high blood pressure, and a greater risk for heart disease.
Trans fat is abundant in fast food restaurants. It is consumed in greater quantities by people who do not have access to a diet consisting of fewer hydrogenated fats, or who often consume fast food2.
Health and trans fats
There are two accepted blood tests that measure an individual’s risk for coronary heart disease.
The first considers ratios of two types of cholesterol, the other the amount of a cellsignalling cytokine called C- reactive protein. The ratio test is more accepted, while the cytokine test may be more powerful. The effect of trans fat consumption has been
documented3,4,5 .
Cholesterol ratio: This ratio compares the levels of LDL to HDL. Trans fat behaves like saturated fat by raising the level of LDL, but, unlike saturated fat, it has the additional adverse effect of decreasing levels of HDL.
The net increase in LDL/HDL ratio with trans fat is approximately double that due to saturated fat. (Higher ratios are worse.).
C-reactive protein (CRP): A study of over 700 nurses showed that those in the highest quartile of trans fat consumption had blood levels of CRP that were 73% higher than those in the lowest quartile6,7,8.
A 2006 study supported by the National Institutes of Health and the USDA Agricultural Research Service concluded that palm oil is not a safe substitute for partially hydrogenated fats (trans fats) in the food industry, because palm oil results in adverse changes in the blood concentrations of LDL and apolipoprotein B just as trans fat does10,11,12,13,14.
Conjugated Linoleic acid (CLA)
Conjugated fatty acids are polyunsaturated fatty acids in which at least one pair of double bonds are separated by only one single bond, as in conjugated linoleic acid, in Fig. 5, whereas in Fig 4 you will notice the three double bonds of alpha-linolenic acid (in red) are separated by one further carbon, i.e. they are not conjugated.
Conjugated linoleic acids (CLA) are a family of at least 28 isomers of linoleic acid found mostly in the meat and dairy products derived from ruminants (Table 1).
One such isomer of CLA is shown in Fig. 5
CLAs are both a trans and a cis fatty acids. The cis bond causes a lower melting point and ostensibly also the observed beneficial health effects. Unlike other trans fatty acids, they may, therefore, have beneficial effects on human health.
In the United States, trans linkages in a conjugated system are not counted as trans fats for the purposes of nutritional regulations and labelling.
CLA and some trans isomers of oleic acid are produced by microorganisms in the rumens of ruminants (Table 1).
Non-ruminants, including humans, produce certain isomers of CLA from trans isomers of oleic acid, such as vaccenic acid (not a conjugated fatty acid, as it possesses only one double bond, Table 1), but, which is converted to CLA by the enzyme, delta-9-desaturase.
Most studies of CLAs have used a mixture of isomers wherein the isomers c9,t11-CLA (rumenic acid) and t10,c12-CLA were the most abundant. More recent studies using individual isomers indicate that the two isomers have very different health effects15,16.
In healthy humans, CLA and the related conjugated linolenic acid (CALA) isomers are bioconverted from linoleic acid and alpha-linolenic acid, respectively, mainly by Bifidobacterium bacteria strains inhabiting the gastrointestinal tract.
This bacterium is considered to produce beneficial effects, especially in babies. In 2008, the United States Food and Drug Administration categorized CLA as generally recognized as safe (GRAS).
Dietary sources of conjugated linoleic acid
Kangaroo meat may have the highest concentration of CLA17. Food products from grass-fed ruminants, e.g. mutton, beef18, dairy products and eggs are good sources of CLA19,20.
Some mushrooms, such as Agaricus bisporus and Agaricus subrufescens, are rare non-animal sources of CLA21.
Health effects of N-6 and N-3 polyunsaturated fatty acids (i.e. LA, ALA, EPA and DHA ) and monounsaturated fatty acids (e.g. oleic
acid)
The relationship between blood levels of cholesterol and cardio-vascular disease (CVD), or that between the intake of SFA and PUFA or MUFA and CVD is somewhat inconclusive.
Investigations have failed to show an effect on flow-mediated dilatation, or other measures of vascular function; whereas significant beneficial effects on blood lipids and slight beneficial effects on blood pressure have been observed by replacing SFA with either MUFA or PUFA, or by a diet high in carbohydrate 22,23,24,25,26,27.
It can be concluded that LC PUFA, (n-6 & n-3) and MUFA do benefit blood lipids when they replace SFA in the diet; but the relationship between this and risk of cardio-vascular disease is less clear.
On balance there seems to be a benefit from the use of unsaturated fatty acids.
However, it should be borne in mind that margarines, where the melting point of unsaturated oils has been raised, may contain undesirable concentrations of trans-fatty acids.
When this occurs the beneficial effect can be nullified. This points to the importance of declarations on labels of the trans-fatty acid content.
The only reservation to this is the conjugated fatty acids of dairy products, brought about by the rumen microbial activity, in which the molecule contains both cis and trans modification in adjacent pairs of C atoms in the carbon chain.
The evidence indicates that conjugated fatty acids are generally recognized as safe.
Health effects of Long chain N-3 fatty acids
The three types of dietary essential omega-3 fatty acids involved in human physiology are a-linolenic acid (ALA) (found in plant oils)28,29,30, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) (both commonly found in marine oils).
A meta-analysis31 showed a positive relationship between dietary cholesterol intake and serum cholesterol concentration; but surveys of any specific relationship between serum cholesterol concentration and the incidence of cardio-vascular disease (CVD) is weak.
The reason for this is that the incidence of CVD is negatively associated with dietary fibre and vegetable protein intake which are also inversely associated with cholesterol intake.
The risk of CVD is correlated with saturated fatty acid intake and the proportion of energy from fat, which in turn is positively associated with cholesterol intake.
Nevertheless, saturated fatty acids are relatively stable in air, whereas foods containing unsaturated fatty acids are vulnerable to oxidation and rancidity when exposed to air if the foods are inadequately protected by natural or synthetic permissible antioxidants.
The results of meta-analyses of surveys and prospective cohort studies indicate that the consumption of fish or fish oil, in which there are high concentrations of tocopherols and other natural antioxidants protecting the n-3 fatty acids, eicosapentaenoic acid (EPA) and
docosahexaenoic acid (DHA), is associated with decreased cardiovascular death, whereas consumption of the vegetable oil-derived n-3 a-linolenic acid is beneficial, but not as effective31,32,33.
Randomized control trials (RCTs) in the context of secondary prevention indicate that the consumption of EPA plus DHA is protective at doses <1 g/d. The therapeutic effect appears to be due to suppression of fatal arrhythmias rather than stabilization of atherosclerotic plaques.
At doses >3 g/d, EPA plus DHA can improve cardiovascular disease risk factors, including decreasing plasma triacylglycerols (triglycerides), blood pressure (systolic and diastolic), platelet aggregation, and inflammation, while improving vascular reactivity33.
Effects of Omega-3 Fatty Acids on Cardiovascular Disease
Mainly on the basis of the results of RCTs, the American Heart Association recommends that everyone eat oily fish twice per week and that those with coronary heart disease eat 1 g/d of EPA plus DHA from oily fish or supplements34.
Owing to the global scarcity of fish oil sources farmed fish at present contain less EPA or DHA, than do wild fish.
This situation, we trust, will be rectified in due course by the development of a genetically modified Camelina
sativa, as a land-based source of EPA and DHA by Haslam et al. (pages15-23).
Inflammation
Some research suggests that the anti-inflammatory activity of long-chain omega-3 fatty acids may translate into clinical effects.
A 2013 systematic review found tentative evidence of benefit35. Consumption of omega-3 fatty acids from marine sources lowers markers of inflammation in the blood, such as C-reactive protein, interleukin 6, and TNF alpha.
For rheumatoid arthritis (RA), one systematic review found consistent, but modest, evidence for the effect of marine n-3 PUFAs on symptoms such as “joint swelling and pain, duration of morning stiffness, global assessments of pain and disease activity”35,36.
However, the American College of Rheumatology has stated that there may be modest benefit from the use of fish oils, but that it may take months for effects to be seen, and cautions for possible gastrointestinal side effects and the possibility of supplements containing mercury or vitamin A at toxic levels37.
Sources of long-chain N-3 fatty acids
The world’s oceans are warming up.
As the mean temperature of oceans rise it decreases their oxygen tension with a likely impact on wild fish stocks.
This fact together with over-fishing to meet the demands of an ever-increasing world human population, is leading to a depletion of these stocks, indicating the essentiality of fish farming.
Fish, squid and krill are a good natural source of N-3 ( -3) fatty acids.
However, these species are unable to manufacture long chain N-3 fatty acids (EPA and DHA) to meet their needs.
They depend on marine algae and phytoplankton as the primary sources of these fatty acids.
These single cell organisms are the only significant source of EPA and DHA, required by both fish and humans, (although several bacterial genera have been identified as sources, e.g. Cellulophaga, Pibocella and Polaribacter in the Antarctic are able to produce EPA & DHA)38.
The future of oceanic phytoplankton is unclear, owing to climate change. There are plant sources of shorter chain N-3 fatty acids e.g. alpha linolenic acid (ALA), including walnut, edible seeds, clary sage seed oil, algal oil, flaxseed oil, Sacha Inchi oil, Echium oil, and hemp oil.
Fish and eventually Man depend on phytoplankton and the algae for the synthesis of long chain N-3 fatty acids in quantities to meet their nutritional requirements.
The ability to transfer phytoplankton genes for the desaturase and elongase enzymes for the synthesis of EPA and DHA from shorter chain a-linolenic acid to false flax, described in this issue by Haslam and colleagues at Rothamsted, offers to potentially meet these needs without further destruction of the marine biosphere.
Omega-3 and omega-9 conclusions
Overall, there is strong evidence that fish oils have a beneficial effect on blood triglycerides (neutral fat) that is dose-dependent and similar in various populations33,39,40.
There is also evidence of a very small beneficial effect of high doses (3-4g/d) fish oils on blood pressure41, especially that of DHA42 and possible beneficial effects on: coronary artery restenosis (stenosis-narrowing of the artery, restricting blood flow) after angioplasty43,44 and on exercise capacity in patients with coronary atherosclerosis, and possibly heart rate variability, particularly in patients with recent myocardial infarctions44, 45.
No consistent beneficial effect is apparent for other analysed CVD risk factors or intermediate markers. However, there is also no consistent evidence of a detrimental effect of omega-3 fatty acids on glucose tolerance. The correlation between intake of omega-3 fatty acids and tissue levels is fairly uniform in different tissues46.
Palm oil (from Elaeis guineensis L.)
In this Issue we publish a paper on palm oil which contains approximately 44% palmitic acid and 37% oleic acid.
As palmitic acid is fully saturated, and in an oil, a liquid at room temperature, it is more stable than many other oils, and so less subject to rancidity; but its saturated nature could have poorer health benefits than oils containing polyunsaturated fatty acids. Furthermore, its production has led to the destruction of vast areas of tropical forest.
Nevertheless, it contains approximately 38% oleic acid, a monounsaturated fatty acid (Table 1), a fatty acid that has some putative health benefits.
Olive oil (from Olea europaea L.)
The Mediterranean Diet is one heavily influenced by monounsaturated fats.
People in countries bordering the Mediterranean consume more total fat than those who live in Northern European countries, but most of the fat is in the form of monounsaturated fatty acids from olive oil and omega-3 fatty acids from fish, vegetables, and certain meats, while consumption of saturated fat is minimal in comparison.
The diet in Crete is fairly high in total fat (40% of total energy, almost exclusively provided by olive oil) yet it affords a remarkable protection from coronary heart disease49 (and probably colon cancer) ,owing, possibly to a very high oleic acid content50 (Tables 1 and 2). Much of the benefit may stem from the use of this oil in its uncooked form by Mediterranean countries.
Although olive oil contains oleic acid at levels of 60-80% a major health benefit is thought to be the non-fat lipids (e.g. 0.2% phytosterol and tocosterols, hydroxytyrosol, oleuropein aglycone, and ligstroside along with traces of squalene (up to 0.7%) present only in the extra virgin oil).
Olive oil is a source of at least 30 phenolic compounds, among which is elenolic acid, a marker for maturation of olives.
The composition of a sample varies by cultivar, region, altitude, time of harvest, and extraction process (Table 2).
Although epidemiological studies indicate that a higher proportion of oleic acid in the diet may be linked with a reduction in the risk of coronary heart disease a comprehensive scientific review by the European Food Safety Authority (EFSA)51 in 2011, concluded cause-and-effect relationships have not been adequately established for consumption of olive oil and for maintaining 1) normal blood LDL-cholesterol concentrations, 2) normal (fasting) blood concentrations of triglycerides, 3) normal blood HDL-cholesterol concentrations, and 4) normal blood glucose concentrations.
But a large scale KANWU study52 found that increasing monounsaturated fat and decreasing saturated fat intake could improve insulin sensitivity, but only when the overall fat intake of the diet was low.
Studies have shown that substituting dietary monounsaturated fat for saturated fat is associated with increased daily physical activity and resting energy expenditure53. More physical activity was associated with a higher-oleic acid diet than one of a palmitic acid diet. In children, consumption of monounsaturated oils is associated with healthier serum lipid profiles54.
Limited and not conclusive scientific evidence suggests that eating about 2 tbsp. (23g) of olive oil daily may reduce the risk of coronary heart disease owing to the monounsaturated fat in olive oil.
To achieve this possible benefit, olive oil is to replace a similar amount of saturated fat and not increase the total daily energy intake. In the United States, producers of olive oil may place a restricted health claim on product labels55.
This decision was announced November 1, 2004, by the Food and Drug Administration after application was made to the FDA by producers. Similar labels are permitted for foods rich in medium chain omega-3 fatty acids such as walnuts and hemp seed. (A Summary of Qualified Health Claims Subject to Enforcement Discretion).
But as mentioned above the low incidence of heart disease associated with a Mediterranean diet may owe, at least in part, to the non-fat, lipid constituents present only in extra virgin olive oil and the use of oil in the uncooked form, rather than as margarine.
Overall Conclusions
1. Vegetable oils are extracted mainly from seeds of: maize, soyabean, sunflower, linseed, rape, olive, and palm. There is concern over the effect of oil palm plantations on the destruction of wild life, reduction in biodiversity and damage to soil composition and structure, as well as the release of greenhouse gases during the initial clearance of the planting area.
2. The yield of oil/ha from the genetically improved oil-palm is very considerably greater than that, for example, of olives.
3. Vegetable oils provide over twice as much net energy/kg compared with starch and so their consumption can contribute to obesity with all its adverse effects on health.
4. Vegetable oils are a source of two dietary essential nutrients in the form of the polyunsaturated fatty acids (PUFA), C18:2, 6, linoleic acid and C18:3, 3, a-linolenic acid. Olive oil is also a rich source of C18:1, 9, oleic acid, a MUFA, which may contribute to its health benefits.
5. At present there has been no vegetable source of long chain omega-3 dietary essential fatty acids C20:5, 3, eicosapentaenoic acid (EPA), and C22:6, 3, docosahexaenoic acid (DHA).
Both these omega-3 fatty acids are essential in the diet of vertebrates, including fish and humans; but the only major source of them is the simple oceanic mono-cellular organisms, phytoplanktons and algae.
Haslam et al. at Rothamsted describe in this Issue, their harvesting from these organisms the group of genes needed for the elongase and desaturase enzymes necessary for the production of EPA and DHA from a-linolenic acid, a C18:3, omega-3 acid.
They have successfully incorporated these genes in the gene pool of Camelina sativa, false flax, a crop which can be grown in temperate climates. This will be an essential source for fish farming where their current source from wild fish oils, is becoming scarce.
6. There is now reasonable evidence that long chain omega-3 dietary essential fatty acids (C20:5, 3 and C22:6, 3), influence risk factors of cardio-vascular disease (CVD).
The best evidence is that they are more potent than are the dietary essential, a-linolenic acid, an omega-3 acid (C18:3 3), linoleic acid an omega-6 acid (C18:2, 6), or than oleic acid, an omega-9 acid (C18:9, 1). All these unsaturated fatty acids are protective, as measured by risk factors to CVD, response; whereas saturated fatty acids, e.g. palmitic acid (C16:0), are considered to promote CVD, in comparison to a low fat diet56. Nevertheless, it should be remembered that palm oil is rich in both palmitic acid and oleic acid (Table 1).
Olive oil contains a larger proportion of oleic acid and less palmitic acid that does palm oil, and is considered to be protective, but the yield per ha of olive oil is far less than that of palm oil which has its environmental consequences! (see Murphy pages 24-34).
Unsaturated fats are prone to oxidation and rancidity, whereas saturated fats are relatively stable and the hydrogenation of unsaturated vegetable oils during the production of margarine leads to cis-trans isomerisation of unsaturated fats.
The trans-isomers have been shown to raise plasma LDL cholesterol levels and to pose a risk for CVD. Hence, legislation has been introduced in the USA, where trans-fat levels must be declared on the labels of appropriate products in the USA.
The relationship of fats to risk factors of CVD is well established and accepted. However the correlation of these risk factors with the occurrence of CVD is less well secure57 owing to the confounding of factors in mixed diets on the response of individuals.
Some clarification of the situation is possible in the analysis of population studies.
References
1 U.S. Food and Drug Administration (2015) The FDA takes step to remove artificial trans fats in processed foods. June 16th. U.S. Department of Health and Human Services. 1-888-INFO-FDA (1-888-463-6332)
2 Anon. Tentative Determination Regarding Partially Hydrogenated Oils; Request for Comments and for Scientific Data and Information A Notice by the Food and Drug Administration on 11/08/2013, Federal Register, The Daily Journal of the United States Government -26854, Vol. 78, (217). Archived from the original on 6 April 2014.
3 Ascherio, A., Katan, M.B., Zock, P.L., Stampfer, M.J. and Willett, W.C. (1999). “Trans fatty acids and coronary heart disease”. New England Journal of Medicine 340 (25): 1994–1998. doi: 10.1056/ NEJM199906243402511. PMID 10379026.
4 Mensink, R.P. and Katan, M.B. (1990). Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. New England Journal of Medicine 323: 439–45. doi :10.1056/NEJM199008163230703
5 Mensink, R.P., Zock, P.L. Kester, A.D.M.and Katan, M.B. (2003). Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a metaanalysis of 60 controlled trials. American Journal of Clinical Nutrition, 77 (5) 1146–55.
6. Hu, F.B., Bronner L, Willett, W.C., Stampfer, M.J. Rexrode, K.M. et al. (2002) Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA, 287 (14), 1815-21.
7. Iso H, Stampfer M.J., Manson J.E. . Rexrode, K.M.and Hu. F.B. (2001) Prospective study of fat and protein intake and risk of intraparenchymal hemorrhage in women. Circulation, 103 (6),856-63.
8 Lopez-Garcia, E., . Schulze , M.B.,Meigs, J.B., . Manson, J.A.E., Rifai, N., Stampfer,M.J., Willett, W.C. and .Hu, F.B. (2005) Consumption of Trans Fatty Acids Is Related to Plasma Biomarkers of Inflammation and Endothelial Dysfunction. Journal of Nutrition, 135 (3) 562-566.
9 Vega-López,S., Ausman, L.M. , Jalbert, S.M., Erkkilä, A.T. and Lichtenstein, A.H. (2006) Palm and partially hydrogenated soybean oils adversely alter lipoprotein profiles compared with soybean and canola oils in moderately hyperlipidemic subjects. Am J Clin Nutr 84 (1) 54-62.
10 Mozaffarian, D., Katan M.B., Ascherio A., Stampfer M.J., Willett, W.C. (2006) Trans fatty acids and cardiovascular disease. N. Engl. J. Med. 354 (15), 1601–13.
11 Kannel, W.B. and Vasan, R.S. (2009) Triglycerides as vascular risk factors: new epidemiologic insights. Current opinion in cardiology 24 (4), 345–50. doi:10.1097/HCO. 0b013e32832c1284. PMC 3012388. PMID 19424059.
12 Danesh, J., Collins, R. and Peto, R. (2000). Lipoprotein(a) and coronary heart disease. Meta- analysis of prospective studies. Circulation,102 (10), 1082–5. doi:10.1161/01.CIR.102.10.1082. PMID 10973834.
13 Smolders, B., Lemmens, R. and Thijs, V. (2007) Lipoprotein (a) and stroke: a meta-analysis of observational studies. Stroke 38 (6), 1959–66. doi:10.1161/STROKEAHA.106.480657. PMID 17478739
14 Schreiner, P.J., Morrisett, J.D., Sharrett, A.R., Patsch, W., Tyroler, H.A., Wu, K. and Heiss, G. (1993) Lipoprotein(a) as a risk factor for preclinical atherosclerosis. (PDF). Arterioscler. Thromb. 13 (6), 826–33. doi:10.1161/01.ATV.13.6.826. PMID 8499402.
15 Funck L.G., Barrera-Arellano, D., Block, J.M. (2006) Conjugated linoleic acid (CLA) and its relationship with cardiovascular disease and associated risk factors. Arch Latinoam Nutr. 56 (2):123-34.
16 Bhattacharya, A., Banu, J., Rahman, M., Causey, J. and Fernandes G. (2006) Biological effects of conjugated linoleic acids in health and disease. J Nutr Biochem. 17 (12):789-810. Epub 2006 May 2.
17 Commonwealth Scientific and Industrial Research Organisation. (2004)-04-23 ”Kangaroo meat – health secret revealed” (Press release). Retrieved, CSIRO 2007-01-23.
18 Dhiman, T.R., Satter, L.D, Pariza, M.W.,. Galli, M.P. Albright, K. and Tolos M.X.(2000). Conjugated Linoleic Acid (CLA) Content of Milk from Cows Offered Diets Rich in Linoleic and Linolenic Acid. Journal of Dairy Science 83 (5), 1016–1027. doi:10.3168/jds.S0022-0302(00) 74966-6. PMID 10821577. Retrieved 2006-05-27
19 Dhiman, T.R. (2001). Role of diet on conjugated linoleic acid content of milk and meat. (PDF). Journal of Animal Science, 79. Retrieved 2007-03-09.
20 Lin Yang, Ying Cao, Zhen-Yu Chen and Cao Chen (2004). Stability of conjugated linoleic acid isomers in egg yolk lipids during frying. Food Chemistry (Elsevier) 86 (4), 531–535.
21 Jang, W.J., Hyung, S.W.(undated) Production of natural c9,t11 conjugated linoleic acid (c9,t11 CLA) by submerged liquid culture of mushrooms. Division of Applied Life Science (BK21), Graduate School, Gyeongsang National University, Jinju, 660-701, South Korea.
22 Keogh, J.B., Grieger, J.A., Noakes, M.& Clifton, P.M. (2005) Flow-Mediated Dilatation Is Impaired by a High–Saturated Fat Diet but Not by a High-Carbohydrate Diet. Arteriosclerosis, Thrombosis and Vascular Biology; 25, 1274-1279.
23 Kris-Etherton,P. M.(1999) Monounsaturated Fatty Acids and Risk of Cardiovascular Disease, Review for the Nutrition Committee. Circulation.100, 1253-1258 doi: 10.1161/01.CIR. 100.11.1253.
24 Richard, D., Bausero ,P.,, Schneider, C .& Visioli, F. (2009) Polyunsaturated fatty acids and cardiovascular disease. Cellular and Molecular Life Sciences 66, (20), 3277-3288.First online: 10 July 2009.
25 Allayee,H., Roth, N. and Hodis, H.N.(2009) Polyunsaturated Fatty Acids and Cardiovascular Disease: Implications for Nutrigenetics. J. Nutrigenet Nutrigenomics, 2 (3), 140–148. Published online 2009 Sep 23. doi: 10.1159/ 000235562 PMCID: PMC2820567
26 Schwingshackl, L. and Hoffmann, G. (2012) Monounsaturated Fatty Acids and Risk of Cardiovascular Disease: Synopsis of the Evidence Available from Systematic Reviews and Meta-Analyses. Nutrients 4 (12), 1989–2007. Published online 2012 Dec 11. doi: 10.3390/nu4121989 PMCID: PMC3546618
27 Frape, D.L., Williams, N.R., Carpenter K, Freeman, M.A., Palmer C.R. and Fletcher, R.J. (2000) Insulin response and changes in composition of nonesterified fatty acids in blood plasma of middle-aged men following isoenergetic fatty and carbohydrate breakfasts. British Journal of Nutrition 84, 737-745.
28 Pan A., Chen M, Chowdhury R, Wu J.H, Sun Q, Campos H, Mozaffarian D, Hu FB (2012) a-Linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr., 96 (6):1262-73. doi: 10.3945/ajcn. 112.044040. Epub 2012 Oct 17.
29 Rodriguez-Leyva, D. Bassett, C.M.C., McCullough, R. and Pierce, G.N. (2010) The cardiovascular effects of flaxseed and its omega-3 fatty acid, alphalinolenic acid. Can J Cardiol. 26 (9): 489–496. PMCID: PMC2989356.
30 Pan A, Chen M, Chowdhury R; et al. (2012). a-Linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis. Am. J. Clin. Nutr. (Systematic review) 96 (6): 1262–73. doi:10.3945/ajcn.112.044040. PMC 3497923. PMID
31 Marchioli, R. and Levantesi, G.(2013) n–3 PUFAs in cardiovascular disease. Proceedings from the Round Table on “Current evidence and future perspectives on n–3 PUFA” London, UK, 2012. International Journal of Cardiology, 170, (2) Supplement 1, S33–S38 Ed. Francesco Pelliccia S0167527313X00361-cov150h.gif
32. Mozaffarian, D.and,Wu, J. H.Y.(2011) Omega-3 Fatty Acids and Cardiovascular Disease : Effects on Risk Factors, Molecular Pathways, and Clinical Events. Journal of the American College of Cardiology, 58, (20), 2047–2067.
33 Anon. Agency for Healthcare Research and Quality ( AHRQ) U.S. Departmentof Health & Human Services (2004) Effects of Omega-3 Fatty Acids on Cardiovascular Disease Evidence Report/ echnology Assessment: Number 94. www.ahrq.gov., www.hhs.gov AHRQ, Archived EPC Evidence Reports, Publication No. 04-E009-2 March 2004
34 Anon. The American Heart Association (2015) Eating fish for heart health, Tweet 29, updated 15th May,2015, 7272 Greenville Ave. Dallas, TX 75231, Customer Service, 1-800-AHA-USA-1, 1-800-242-8721
35. Di Giuseppe, D., Wallin, A. Bottai, M., Askling, J. and Wolk, A. (2013) Long-term intake of dietary long-chain n-3 polyunsaturated fatty acids and risk of rheumatoid arthritis: a prospective cohort study of women Ann Rheum Dis doi:10.1136/ annrheumdis-2013-203338 Clinical and epidemiological research
36 Calder PC (2008). Symposium on ‘Nutrition and autoimmune disease’ PUFA, inflammatory processes and rheumatoid arthritis. Proc Nutr Soc. 67 (4), 409-18. doi: 10.1017/S0029665108 008690. Session 3: Joint Nutrition Society and Irish Nutrition and Dietetic Institute
37 Geusens P., Wouters, C. Nijs, J.,Jiang Y. and Dequeker J.(2005) Long-term effect of omega-3 fatty acid supplementation in active rheumatoid arthritis Arthritis & Rheumatism 37, (6), 824–829, Article first published online: 9 DEC 2005 DOI: 10.1002/art.1780370608 Copyright © 1994 American College of Rheumatology.
38 Bianchi, A. C., Olazábal, L, Torre, A. and Loperena, L (2014) Antarctic microorganisms as source of the omega-3 polyunsaturated fatty acid. World Journal of Microbiology and Biotechnology. 30 (6) 1869-1878. First online: 29 January 2014.
39 Svensson M., Christensen J.H., Sølling J., Schmidt E.B.(2004) The effect of n-3 fatty acids on plasma lipids and lipoproteins and blood pressure in patients with CRF. Am J Kidney Dis. 44 (1) 77-83.
40 Boberg M., Pollare, T., Siegbahn, A., Vessby, B. (1992) Supplementation with n-3 fatty acids reduces triglycerides but increases PAI-1 in non-insulindependent diabetes mellitus. Eur J Clin Invest. 22 (10), 645-50.
41. Massaro, M., Scoditti, E., Carluccio M.A., Campana M.C. and De Caterina, R. (2010) Omega-3 fatty acids, inflammation and angiogenesis: basic mechanisms behind the cardioprotective effects of fish and fish oils. Cell Mol. Biol. (Noisy-le-grand). 56, (1), 59-82.
42 Mori T.A., Bao D.Q., Burke V, Puddey I.B. snd Beilin L.J. (1999) Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension. 34 (2), 253-60.
43 Dehmer, G.J., ,Popma,J.J.,van den Berg, E.K.., Eichhorn, E.J.,Prewitt, J.B.,. Campbell, W.B. et al.., (1988) Reduction in the Rate of Early Restenosis after Coronary Angioplasty by a Diet Supplemented with n–3 Fatty Acids. N Engl J Med, 319, 733-740. DOI: 10.1056/NEJM1988092 23191201
44 Balk, E.M, , Lichtenstein, A.H., Chung, M, Kupelnick, B., Chew P and Lau, J. (2006) Effects of omega-3 fatty acids on coronary restenosis, intima– media thickness, and exercise tolerance: A systematic review. Atherosclerosis 184 ,(2), 237– 246.
45 Anon. (2004) U.S. Department of Health & Human Services, www.hhs.gov; Agency for Healthcare Research and Quality AHRQ, Archived EPC Evidence Reports Effects of Omega-3 Fatty Acids on Cardiovascular Risk Factors and Intermediate Markers of Cardiovascular Disease Evidence Report/Technology Assessment: Number 93
46 Garneau,V., Rudkowska, I,, Paradis, A.M., Godin, G., Julien, P., Pérusse, L., and Vohl, M.C. (2012) Omega-3 fatty acids status in human subjects estimated using a food frequency questionnaire and plasma phospholipids level. Nutr J.,11, (46), doi: 10.1186/1475-289.
47 Anon. (1976) online edition: Fat Content and Composition of Animal Products, Nutrient database, Release 24. United States Department of Agriculture. Publishing Office, National Academy of Science, Washington, D.C., ISBN 0-309-02440-4; p. 203.
48 Friesen, R., and Innis, S.M. (2006) Trans Fatty acids in Human milk in Canada declined with the introduction of trans fat food labeling, J. Nut., 136, 2558-2561).
49. Guasch-Ferré, M., Hu, F.B., Martínez-González, M.A., Fitó, M., Bulló,M, Estruch,R, Ros, E., Corella D. et al, (2014) Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Medicine, 12, (78) doi:10.1186/1741-7015-12-78
50 León, L., Uceda, M., Jiménez, A., Martín, L.M. and Rallo, L. (2004) Variability of fatty acid composition in olive (Olea europaea L.)
progenies. Spanish Journal of Agricultural Research 2 (3), 353-359
51 Anon. Scientific Committee/Scientific Panel of the European Food Safety Authority. (2011) Scientific Opinion on the substantiation of health claims related to olive oil and maintenance of normal blood LDL-cholesterol concentrations EFSA Journal (European Commission) 9 (4), 2044 [19 pp]. doi:10.2903/j.efsa.2011.2044. Retrieved April 5, 2013.
52 Vessby B, Uusitupa M, Hermansen K, Riccardi G, Rivellese A .A, Tapsell L .C, Nälsén C, Berglund L, Louheranta A, Rasmussen B .M, Calvert G .D, Maffetone A, Pedersen E, Gustafsson I .B, Storlien L .H. (2001) Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia,.44 (3), 312-9.L, Louheranta A, Rasmussen B .M, Calvert G .D, Maffetone A, Pedersen E, Gustafsson I .B, Storlien L .H. (2001) Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia,.44 (3), 312-9.
53 Kien, C.L., Bunn, J.Y., Tompkins, C.L., Dumas, J.A., Crain, K.I., Ebenstein, D.B., Koves, T.R. and Muoio, D.M. (2013). Substituting dietary monounsaturated fat for saturated fat is associated with increased daily physical activity and resting energy expenditure and with changes in mood. The American Journal of Clinical Nutrition 97 (4), 689–697. doi:10.3945/ajcn.112.051730. PMID 23446891).
54 Sanchez-Bayle M., Gonzalez-Requejo A., Pelaez M.J., Morales M.T., Asensio-Anton J. and Anton-Pacheco E. (2008). A cross-sectional study of dietary habits and lipid profiles. The Rivas-Vaciamadrid study. Eur. J. Pediatr. 167 (2), 149–54. doi:10.1007/s00431-007-0439-6. PMID 17333272.).
55 U.S. Food and Drug Administration (2004) Monounsaturated Fatty Acids From Olive Oil and Coronary Heart Disease. Summary of Qualified Health Claims Subject to Enforcement Discretion. Docket No. 2003Q-0559 11/01/2004 enforcement discretion letter.
56 Frape, D.L., Williams, N.R., Rajput-Williams, J. Maitland, B.W., Fletcher, R.J. and Palmer, C.R. (1998). Circadian variation in blood thrombogenic characteristics in middle-aged healthy men given isoenergetic carbohydrate and fatty breakfasts. Fibrinolysis & Proteolysis, 12, 311-319.
57 Rizos, E.C., Ntzani, E.E., Bika, E., Kostapanos, M.S. and Elisaf, M.S. (2012) Association Between Omega-3 Fatty Acid Supplementation and Risk of Major Cardiovascular Disease Events: A Systematic Review and Meta-analysis. JAMA, 308
(10),1024-1033. doi:10.1001/2012.jama.11374.
Download pdf
Figures
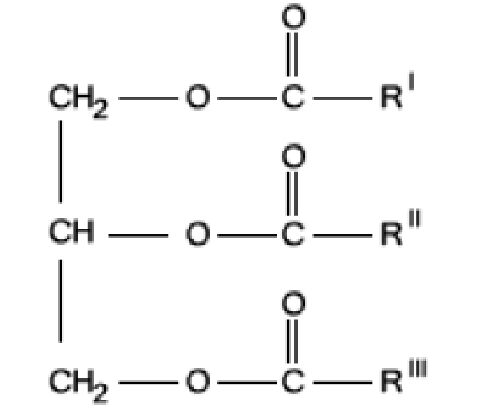
Fig. 1 A triglyceride is a compound derived from glycerol and three fatty acid alkyl chains, R1, R11 and R111, forming an ester through their acyl group. These three chains may be identical, or each one may have a different formula.
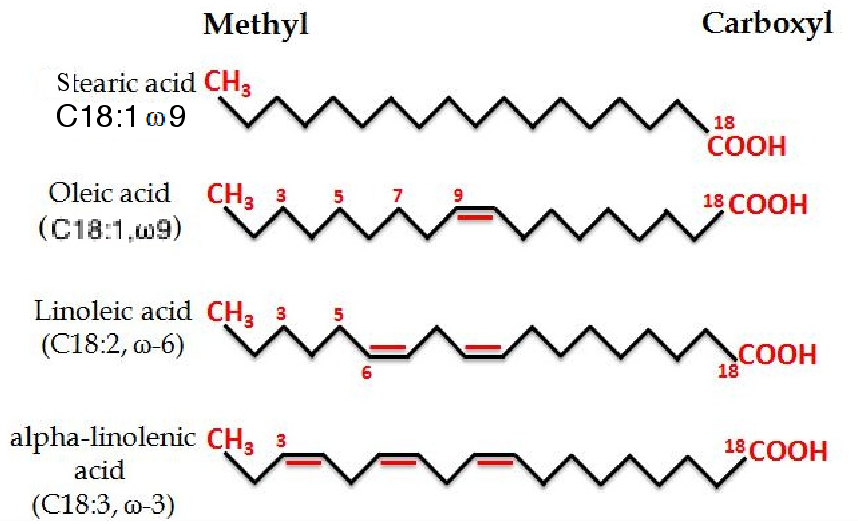
Figure 2 The structural formulae of four fatty acids, the lower two of which are dietary essential fatty acids, in that mammals and fish are incapable of their synthesis from non-essential fatty acids. n.b. The methyl group is at the omega end of the chain, regardless of its length (also known as the n end) and the carboxyl group is at the alpha end.
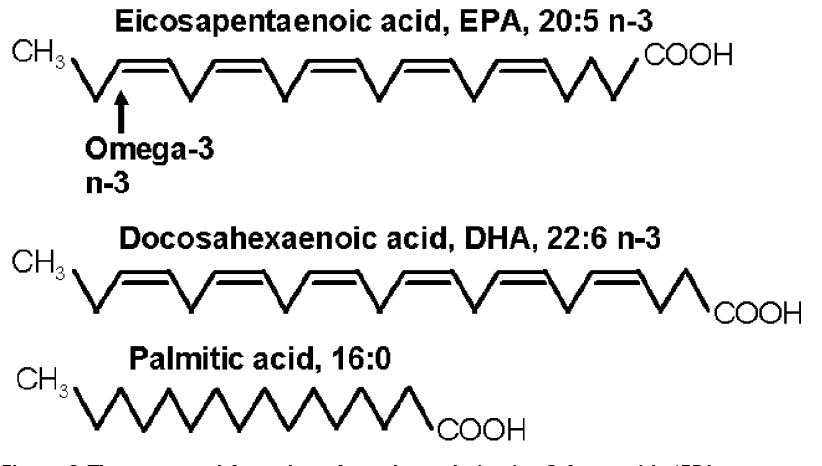
Figure 3 The structural formulae of two long chain cis 3 fatty acids (EPA & DHA), essential in the diet of vertebrates, including fish and of Man. The third is a saturated fatty acid rich in palm oil.
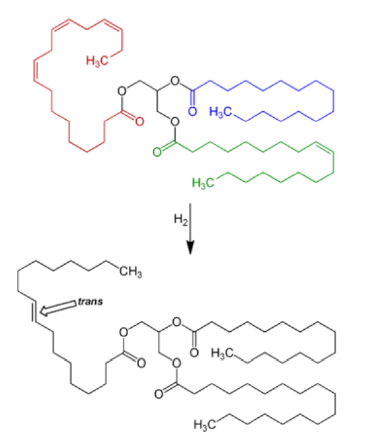
Fig. 4 Reaction scheme for three fatty acids in a molecule of a triglyceride (a triacylglycerol): of trans fat during the hydrogenation process for the production of margarine from oils. Up to 45% of the total fat may contain trans fatty acids. The Figure shows a triglyceride containing in one saturated fatty acid, palmitic acid (blue), one mono-unsaturated fatty acid, oleic acid (green), and one polyunsaturated fatty acid, a-linolenic acid, (red). The latter is changed to a trans.oleic acid* (black), the blue remains palmitic acid; whereas the monounsaturated fatty acid (green) becomes saturated stearic acid (black).*see table 1 for another C18:1 acid, Vaccenic acid.
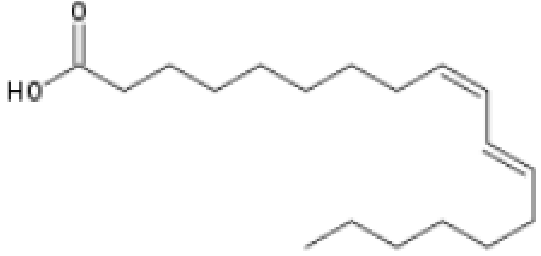
Fig. 5 Conjugated Linoleic acid which has adjacent trans7- and cis9-
bonds.
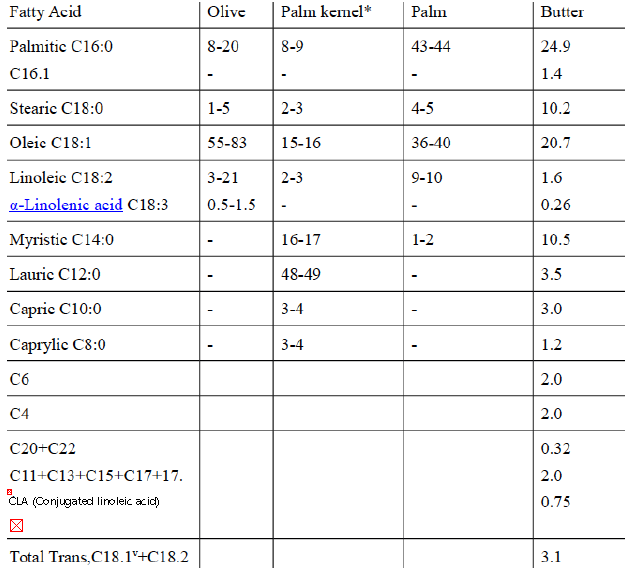
Table 1 Chemical composition of vegetable oils and butter (Moles %)47
Footnotes
* Palm kernel oil is obtained from the kernel of the oil palm fruit.
Vaccenic acidv, also known as (E)-Octadec-11-enoic acid is a C18.1 naturally occurring trans-fatty acid found in the fat of ruminants and in dairy products such as milk, butter, and yogurt. It is also the predominant fatty acid comprising trans fat in human milk48.
The name was derived from the Latin vacca (cow).
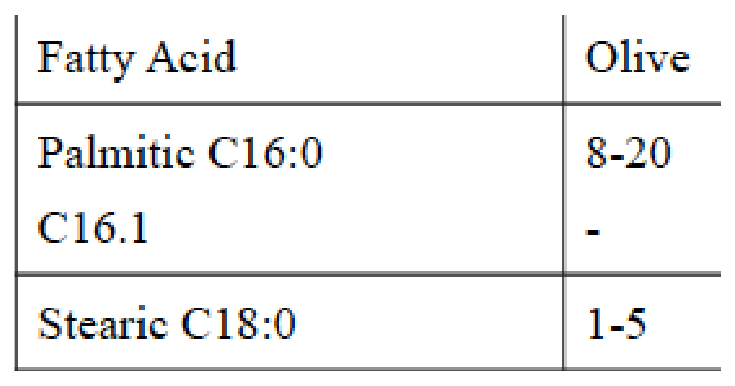
Table 2 Range in fatty acid composition of olive oil

